With Hans-Ruedi Pfeifer (Hon. Prof. at the University of Lausanne), I had the pleasure to publish a paper in the Bulletin de la Société Vaudoise des Sciences Naturelles on the chemical composition of alpine spring waters. This paper is a review of water analysis (major and trace elements) according to the type of bedrock forming the catchment.
Although it is published in French, an extended abstract in English is available and reproduced below, with references to the most significant figures.
Derron M.-H., Pfeifer H.-R (2017) : Caractérisation hydrogéochimique des eaux de source alpines. Bull. Soc :Vaud. Sc.Nat, 96, 5-29.
https://www.e-periodica.ch/digbib/view?pid=bsv-002:2017:96#10
Extended abstract
In order to investigate the influence of bedrock on the chemical composition of alpine spring waters, more than 700 chemical analyses for major and trace elements have been collected from regional reports or thesis. All these waters are from shallow aquifers (no deep or geothermal circulation), where water is cold and oxic, with pH neutral to basic. Five types of bedrock have been distinguished: granite, mafite, ultramafite, limestone and gypseous rocks (mostly gypseous dolomite). Classical physicochemical parameters (pH, temperature and electrical conductivity), major elements and, depending on the authors, about 15 trace elements are usually provided. The concentration ranges of each element in solution, for each type of bedrock, are provided as percentiles in annexes (online). These values are indicators of common water compositions encountered in moderate to high altitude alpine environment.
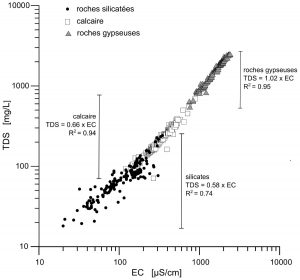
Results for major elements show that the total dissolved load depends directly on the nature of the bedrock: silicated, carbonated or sulfate-bearing rocks (Figure 1).
Figure 1: Total dissolved solid vs electrical conductivity for alpine waters from silicated rocks, limestones and gypseous rocks (N=696).
Classical diagrams of Schoeller (Figure 2) and Piper (Figure 3), as well as the hydrogeochemical facies of JAECKLI (1970), are used to characterize each water type, corresponding to the five types of rocks considered.
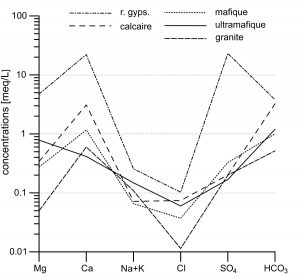 Figure 2: Schoeller’s diagram for the five types of alpine waters considered in this work (median concentrations for each type).
Figure 2: Schoeller’s diagram for the five types of alpine waters considered in this work (median concentrations for each type).
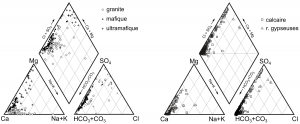 Figure 3: Piper’s diagram of alpine spring waters for granite (N=98), mafic (61) and ultramafic (36) types on the left, limestones (294) and gypseous rocks (207) on the right.
Figure 3: Piper’s diagram of alpine spring waters for granite (N=98), mafic (61) and ultramafic (36) types on the left, limestones (294) and gypseous rocks (207) on the right.
Two types of water are well differentiated from the others. Waters of gypseous rocks are strongly enriched in Ca, Mg and SO4, with SO4/HCO3 >1. Waters from ultramafic rocks are enriched in Mg, with usually Mg/Ca>1. In all the other types of water (from granites, mafites or limestones), Ca and HCO3 strongly dominate. This convergence of compositions towards an undifferentiated calco-hydrogenocarbonated facies is known in metamorphic rocks. It can be attributed to traces of calcite in the silicate rocks and that metamorphic silicate minerals are much less reactive than calcite. In order to improve the discrimination of these water types, a new ternary diagram is proposed, using relative Ca, Mg and Si concentrations (Figure 4).
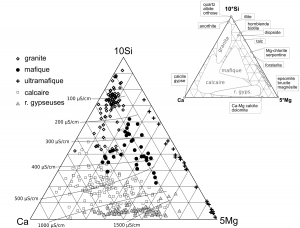 Figure 4: Ca-5Mg-10Si ternary diagram (mMole/L) for alpine spring waters (N=442), with indicative isolines of electrical conductivity. The positions of main rock forming minerals are in the upper figure.
Figure 4: Ca-5Mg-10Si ternary diagram (mMole/L) for alpine spring waters (N=442), with indicative isolines of electrical conductivity. The positions of main rock forming minerals are in the upper figure.
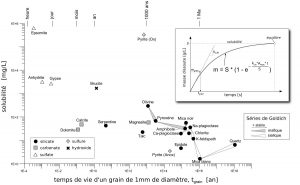
It appears from these analyzes that dissolution properties of minerals (i.e. solubility and dissolution rate) strongly control the content in major elements of these spring waters (Figure 9). In particular, a low amount of a highly soluble and rapidly dissolved mineral may play the main role: gypsum or anhydrite in gypseous rocks, brucite in ultramafites, or calcite in the other rock types.
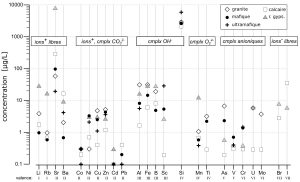
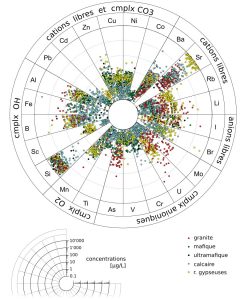
Dissolved contents of trace elements are highly variables, several orders of magnitude for most of them. Median values and overall distributions, by type of rocks, are displayed in Figure 5 and Figure 6 respectively.
Figure 5: Median concentrations of trace elements in alpine spring waters by type of rocks and by valence. Speciation according to Stumm & Morgan 1996 (cmplx = aqueous complexe).
Figure 6: Wheel of trace elements in solution (inside a slice , the points are spread randomly on the radius that corresponds to the concentration).
For most of trace elements, there is no obvious relationship between rock contents and concentrations in solution. Some exceptions are: 1) water from gypseous rocks are enriched in Li, Rb and Sr; 2) concentrations of U, Mo, As are higher in water from granite. In order to interpret these data and to identify the processes regulating the concentrations of trace elements in solution, the valence, the speciation and a mobility index are used (Figure 10). Dissolution properties of minerals seem to control the concentrations of alkaline elements (Li, Rb, Sr, Ba). Very low concentrations of dissolved Fe, Al, Mn and Ti may be due to precipitation as oxy-hydroxides. Adsorption of transition metals (Co, Ni, Cu, Zn, Cd, Pb) on mineral surfaces or suspensions can regulate their concentrations in these basic waters. Higher valence elements (Si, U, Mo, Cr, As) form anionic complexes in natural waters. If they are present in soluble minerals, these anionic complexes may explain the observed enrichment of these elements in some specific types of water (granitic and gypseous).
Figure 9: Solubility vs dissolution rate for main rock forming minerals (pure water at 25°C and equilibrium with atmospheric CO2 and O2). The dissolution rate is expressed as lifetime of a 1mm diameter spherical grain. Both Goldich’s sequences are shown for silicates. The upper figure illustrates a typeical dissolution experiment, with: m = mass of dissolved mineral, kcin = dissolution rate at the beginning of the reaction [g/m2/s], S = solubility [g/L], Areac= reactive surface of the mineral [m2/L].
Figure 10: Ratio of the median concentration in water on the concentration in rock (molar concentrations for both). The higher is this ratio, more the element is mobile in the system.