Paper published in french with an extended abstract in english reproduced below.
Reference: Sahli V. & Derron M.-H. 2019, Hydrogéochimie des eaux minérales suisses en bouteille. Bulletin de la Société Vaudoise des Sciences Naturelles 98: 35-52.
https://www.e-periodica.ch/digbib/view?pid=bsv-002:2019:98::40
In order to investigate the influence of various bedrock types, 59 chemical analyses of Swiss bottled waters were collected by reading the bottle labels or by contacting the producers. The analyses include major cations and anions and usual physicochemical parameters when available (pH, temperature and total dissolved solids). All the springs or wells corresponding to these waters were accurately mapped and the geological context (type of bedrock forming the aquifer) was reported from the 1:25’000 national geological atlas (table 1, figure 1).
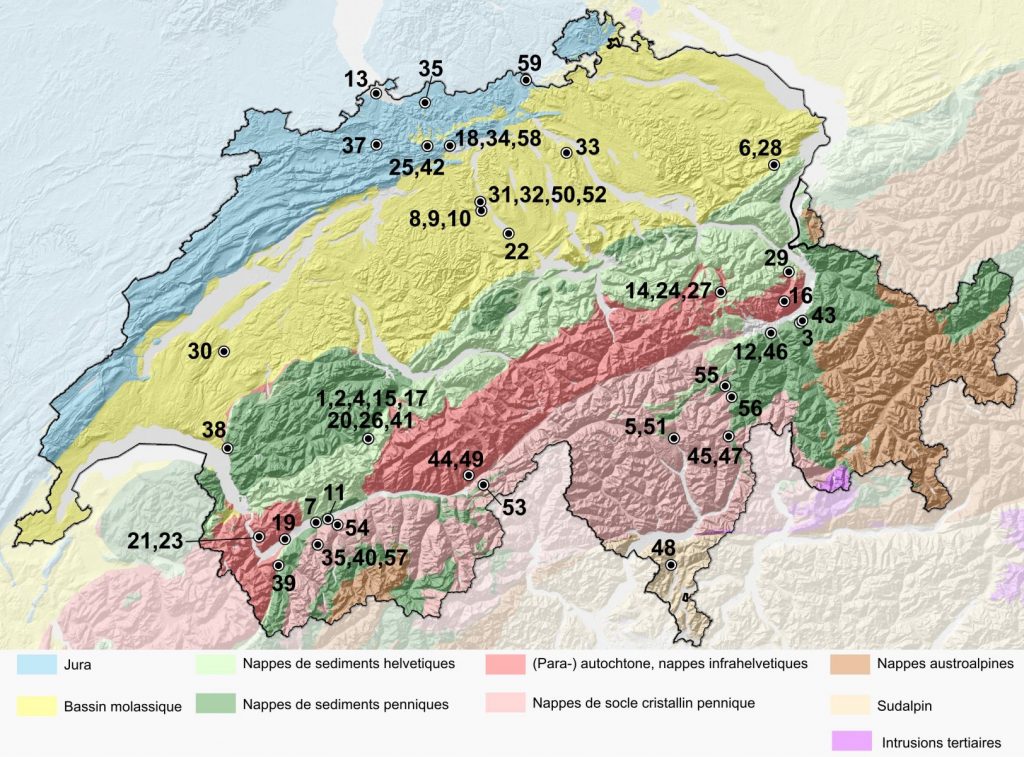 Figure 1. Simplified Swiss tectonic map (Swisstopo, 2019) and location of the 59 studied mineral waters.
Figure 1. Simplified Swiss tectonic map (Swisstopo, 2019) and location of the 59 studied mineral waters.
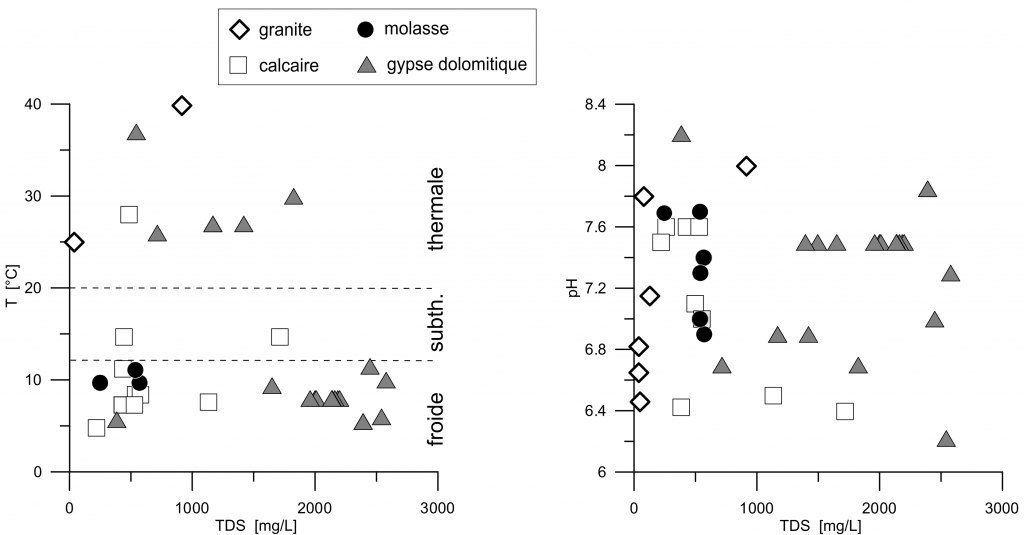
40 waters are from the Alps or Prealps, 12 from the Plateau and 7 from the Jura mountain range. This distribution is indicative as some waters extracted at the same location are sold with different brand names (figure 1). 26 waters are issued from an aquifer in gypseous dolomites, 7 in granite/gneiss, 16 in limestone and 10 in molasse (table 1, and supplementary material on-line). Temperature is known for 41 waters : 29 are cold (T < 12 °C), 2 subthermal (12 °C < T < 20 °C) and 10 thermal (T > 20 °C) (figure 2).
Figure 2. Temperature and pH as a function of Total Dissolved Solids (TDS).
pH ranges from 6.2 to 8.2 and there is no obvious relationship between pH and TDS or geology. TDS is high for waters in contact with gypseous dolomites (>1000 mg/L), intermediate for waters issued from limestone or molasses (200-700 mg/L), and low from waters circulating in granite/gneiss (<200 mg/L).
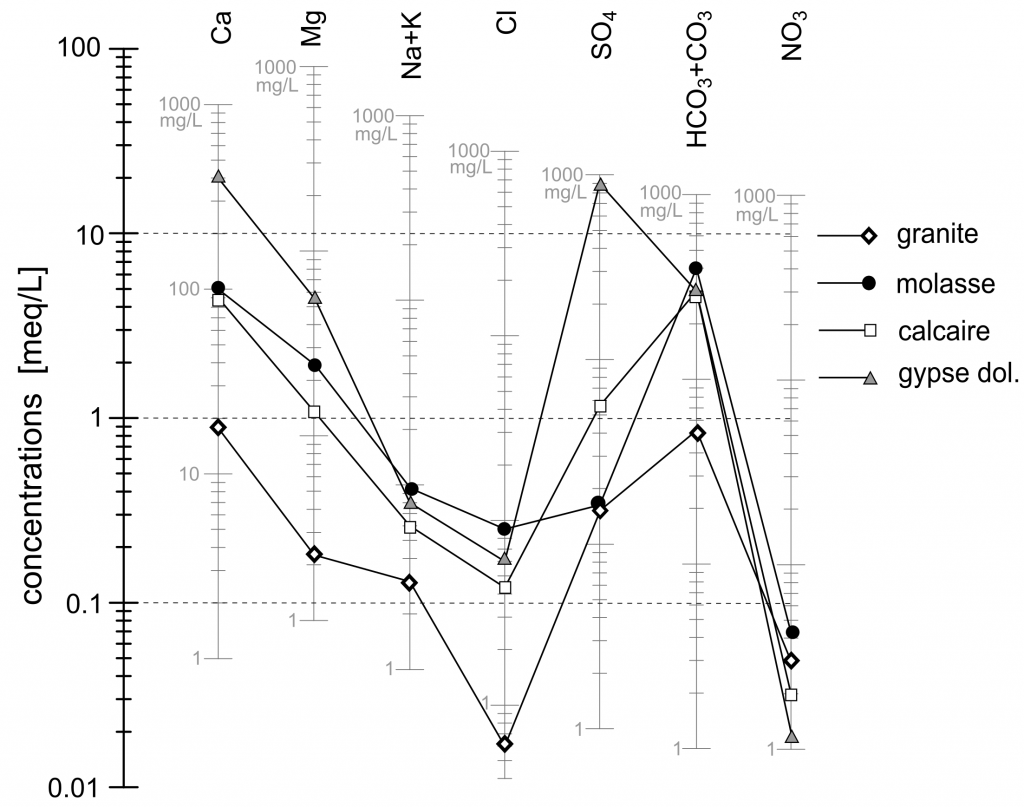
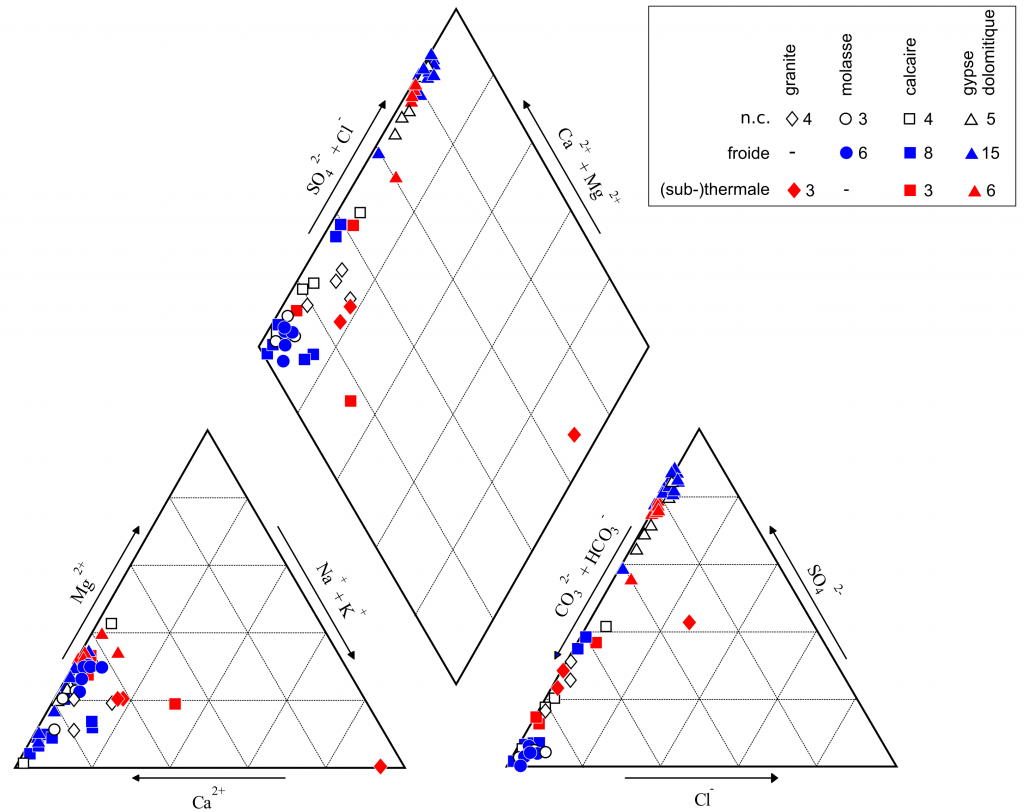
Classical tools of hydrogeochemistry are used to characterize these waters : 1) Schoeller diagram for the absolute concentrations (figure 3), 2) Piper diagram and Jaeckli’s facies for the relative concentrations (figure 4, table 1).
Figure 3. Schoeller Diagram with median values for each type of water.
Figure 4. Piper diagram, as a function of geology and temperature.
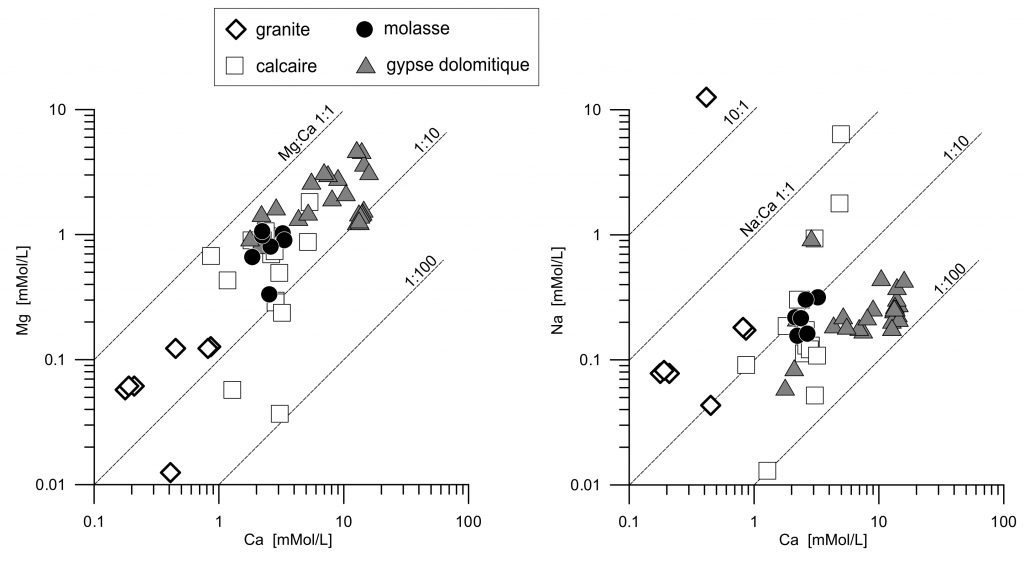
Calcium is the dominant cation in solution, much more abundant than Na or Mg, in all the waters except two that underwent ionic exchange leading to a higher sodium concentration (figure 5).
Figure 5. Solution concentrations of Mg and Na as a function of Ca.
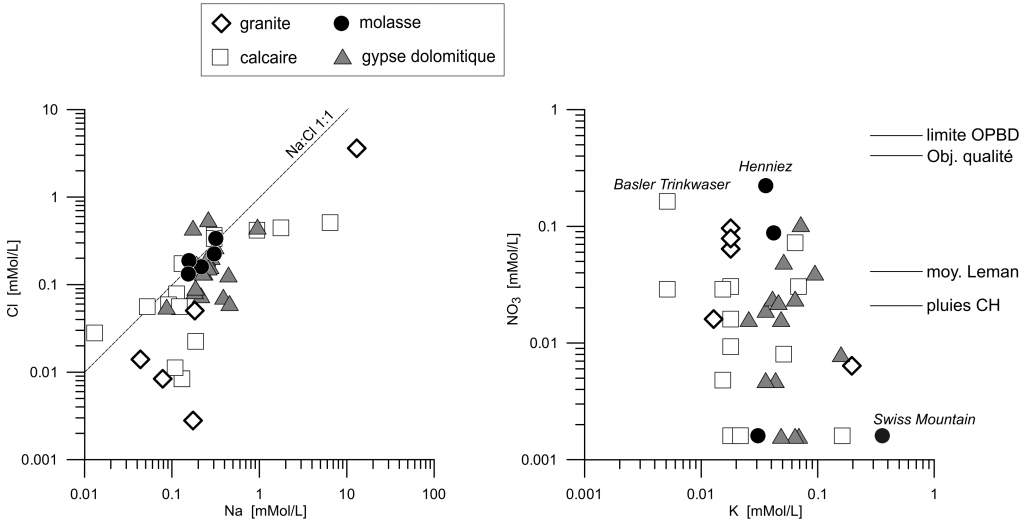
Concentration of Ca is limited by gypsum solubility when gypsum is present, and by calcite and CO2 partial pressure otherwise (figure 7). Five waters are relatively rich in Na and Cl, indicating ionic exchange with clays (figure 6). From the whole list, two of them have nitrate concentrations higher than 10 mg/L, which is still well under the legal limitations (figure 6).
Figure 6. Solution concentrations of Na as a function of Cl, and NO3 as a function of K. Limite OPBD = maximum concentration for Swiss drinking water (=40 mg/L). Obj. qualité = quality objective for Swiss drinking water (=25 mg/l).
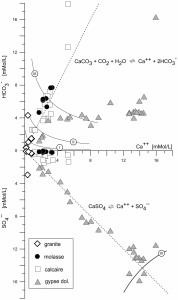
Figure 7. Solution concentrations of HCO3 and SO4 as a function of Ca. Equations and straight lines correspond to the dissolution of calcite and gypsum under surface conditions. Curves I to III correspond to calcite saturation for a partial pressure of
standard atmospheric CO2 (pCO2atm = 10-3.5atm), 10 x pCO2atm and 100 x
pCO2atm (at 12 °C) respectively. Curve IV corresponds to gypsum saturation
at 12 °C.
In general, the imprint of aquifer bedrock is well marked on the chemical composition
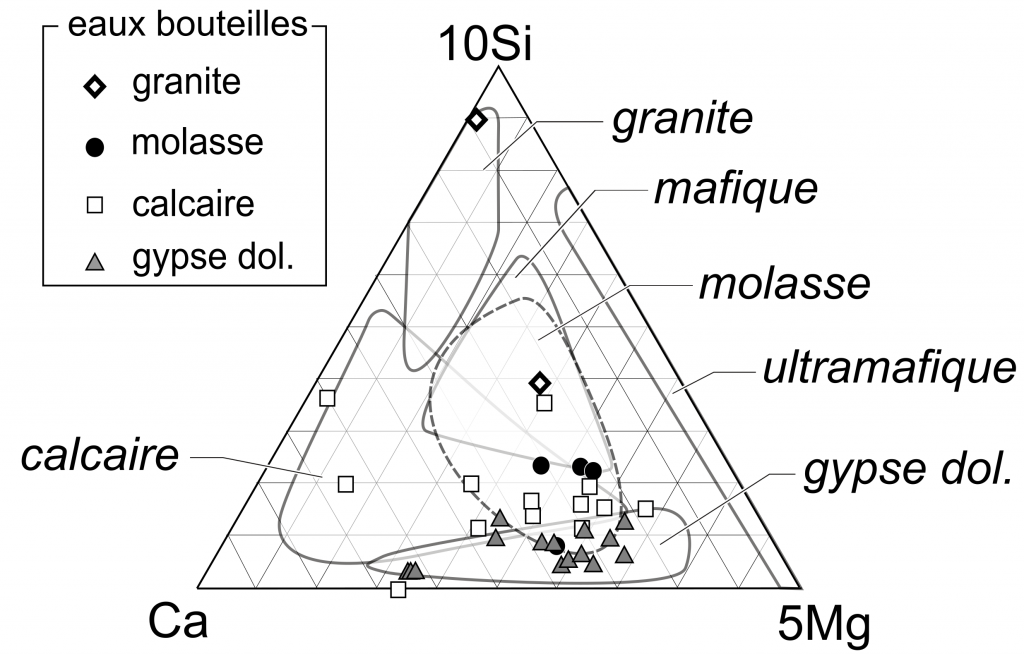
of these waters,, mostly in terms of sulphate, calcium or sodium concentrations. Temperature is of secondary importance. These commercial mineral waters are not significantly different from usual alpine spring waters (figure 8), with respect to the bedrock type. Interestingly a relatively high number of gypsum-related waters are proposed on the market (26 of 59), whereas gypseous rocks are of limited spatial extents in the Swiss Alps. This statement is due to former laws specifying that only highly mineralized waters (TDS > 1000 mg/L) could be commercialized as mineral water. Such high dissolved solid contents are reached only by waters in contact with gypseous dolomites of evaporitic origin in Switzerland.
Figure 8. Typological ternary diagram of spring waters. Symbols represent mineral waters, fields represent area defined by different rock types of alpine spring waters (Derron & Pfeifer 2017) and spring waters from the molasse (analyses compiled from Hesske 1995).