Welcome to the Vještica Lab

Research overview
This section discusses the general topic we are interested in. For more detailed project descriptions, please visit our Research page.
Introduction
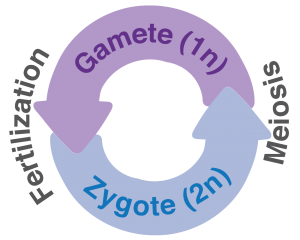 Figure 1: Ploidy cycle
Figure 1: Ploidy cycle
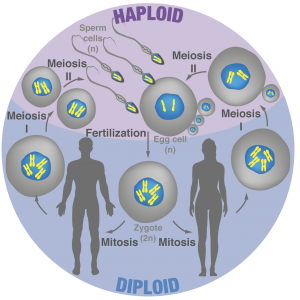 Figure 2: Overview of sexual reproduction in animals
Video 1: Polyspermy block. The large egg cell is fertilized at 2’oclock-position by a tiny sperm cell. Sperm entry initiates Ca2+ wave (righ panel) that leads to formation of a protective layer around the zygote (left panel).
Video 2: Fertilization trigers zygote development.
Figure 2: Overview of sexual reproduction in animals
Video 1: Polyspermy block. The large egg cell is fertilized at 2’oclock-position by a tiny sperm cell. Sperm entry initiates Ca2+ wave (righ panel) that leads to formation of a protective layer around the zygote (left panel).
Video 2: Fertilization trigers zygote development.
How fertilization triggers development is one of the most basic and longstanding question in biology. Indeed, fusion between the sperm and egg transforms these terminally differentiated gametes into a totipotent zygote that gives rise to a new individual (Video 2). A critical but poorly understood step in early development is transcriptional “awakening” of the zygotic genomes. Mechanisms that initiate zygotic gene expression remain unclear, yet homeodomain family of transcription factors plays prominent roles in animals, plants and fungi. Interestingly, zygotic transcription often initiates from one parental genome, which in some instance results in non-Mendelian inheritance of traits from only one parent.
Fission yeast sexual lifecycle
We recently found that the fission yeast sexual lifecycle has surprising similarities with more derived eukaryotes such as plants and animals. We showed that diploid formation in fission yeast confers both meiotic proficiency and mating repression, the two defining properties of zygotic cell fate. Specifically, we observed that gametes not only compete for partners (Video 3) but that zygotes have highly effective re-fertilization blocks, which were previously completely overlooked in fungi. Our current work shows that fungi may prevent consecutive fertilization events through several mating blocks, which we now aim to understand at the molecular level. While it is known that a homeodomain transcription factor serves as the initial trigger, how zygotic signaling propagates to establish the new cell fate remains to be seen. Interestingly, we discovered that zygotes can asymmetrically transcribe the two parental genomes, and consequently, allocate functionality to one parental allele. Our ongoing work aims to identify the prevalence and roles of parental bias in gene expression using fission yeast. This work holds potential to introduce a powerful model system, where experimental evolution is feasible, into the field of parent-specific allelic expression.
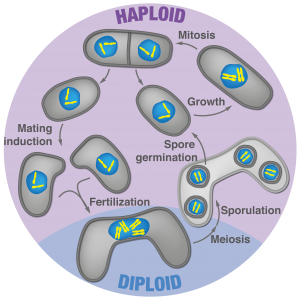 Figure 3: Fission yeast lifecycle
Video 3: Multiple fission yeast cells compete for a partner during mating.
Figure 3: Fission yeast lifecycle
Video 3: Multiple fission yeast cells compete for a partner during mating.