September 2025
Two new videos on topics related to environmental crises (in French)
In the first video, Thimothée Parrique, researcher in ecological economics, explains the concept of degrowth
In the second video, Colin Pahlish,who leads the STRIVE project at UNIL, discusses the importance of narratives in driving the transition toward political and economic systems that are ecologically and socially sustainable
August 2025
New podcast about our research
If you want to know more about the ways CPPs enter cells and why the research on this topic is interesing please listen to this podcast.
June 10-12, 2024
Ali, Francisco, and Christian presented the lab’s work at the GFPP meeting in Montpellier






October 3, 2023
Ali enjoys the prize he got at one of our journal clubs: a concert of the Post Modern Jukebox at the Docks

September 2023
Francisco gets the Best Poster Award at the GFPP meeting

Ali, Francisco, Evgeniya, and Christian at the GFPP meeting in Auvergne (France)
November 2022
Ali, Francisco and Christian went to see Porcupine Tree in Zürich


Evgeniya Trofimenko is back in our lab after a successful postdoc in Paris

October 2022
Christian is an invited speaker at the 60th Annual Meeting of the Biophysical Society of Japan

September 2022
Christian is an invited speaker at the 36th European Peptide Symposium/12th International Peptide Symposium

May 2022 – Two new movies available on youtube highlight some of our recent findings. Check them out
SNF grant obtained (April 2022) ~900’000 CHF

We are happy to announce that we are now supported by a grant from the Swiss National Science Foundation for the next 4 years
Detailed characterization of the CPP translocation pore (December 2, 2021)
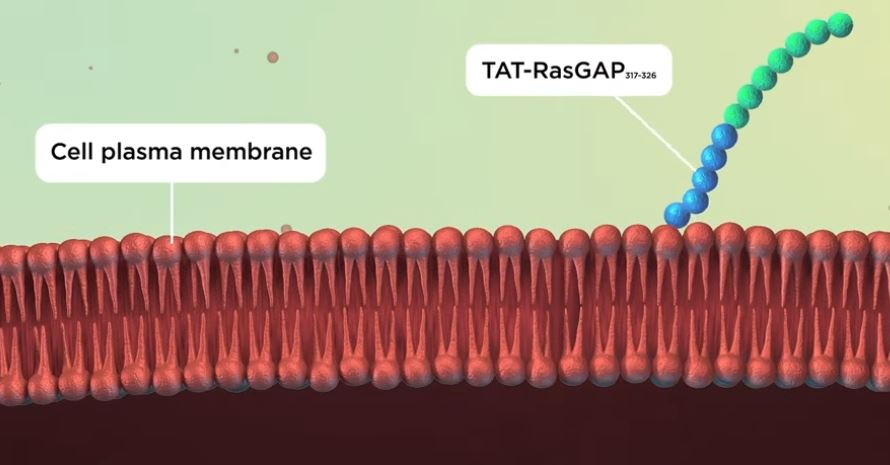
Before a drug can have its desired effect, it must reach its target tissue or organ, and enter its cells. This is not easy because cells are surrounded by the plasma membrane, a fat-based barrier that separates the cell from its external environment. The plasma membrane contains proteins that act as channels, shuttling specific molecules in and out of the cell, and it also holds charge, with its inside surface being more negatively charged than its outside surface.
Cell-penetrating peptides are short sequences of amino acids (the building blocks that form proteins) that carry positive charges. These positive charges allow them to cross the membrane easily, but it is not well understood how.
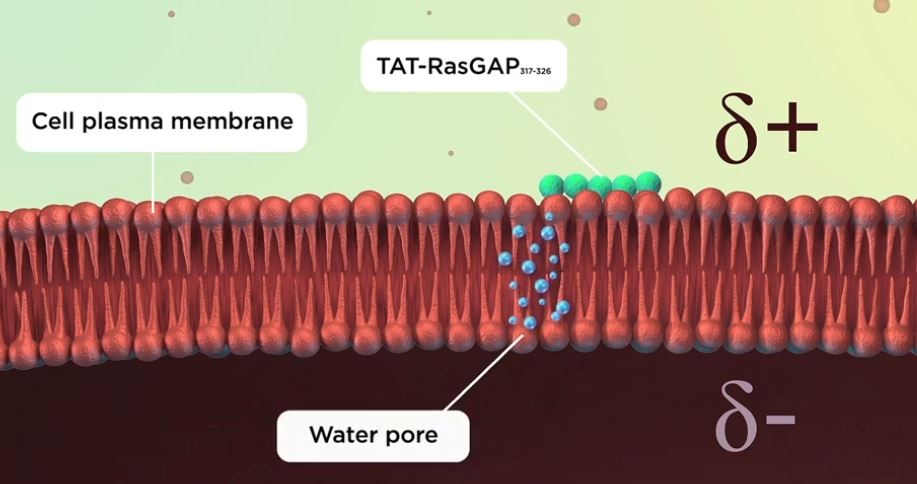
To find out how cell-penetrating peptides cross the membrane, Evgeniya Trofimenko attached them to dyes of different sizes. This revealed that the cell-penetrating peptides enter the cell through temporary holes called water pores, which measure about two nanometres across. The water pores form when the membrane becomes ‘megapolarized’, this is, when the difference in charge between the inside and the outside of the membrane becomes greater than normal. This can happen when the negative charge on the inside surface or the positive charge on the outer surface of the membrane increase. Megapolarization depends on potassium channels, which transport positive potassium ions outside the cell, making the outside of the membrane positive. When cell-penetrating peptides arrive at the outer surface of the cell near potassium channels, they make it even more positive. This increases the charge difference between the inside and the outside of the cell, allowing water pores to form. Once the peptides pass through the pores, the charge difference between the inside and the outside of the cell membrane dissipates, and the pores collapse.
Drug developers are experimenting with attaching cell-penetrating peptides to drugs to help them get inside their target cells. Currently there are several experimental medications of this kind in clinical trials. Understanding how these peptides gain entry, and what size of molecule they could carry with them, provides solid ground for further drug development.
Discovery of a new endocytic pathway (November 2, 2021)
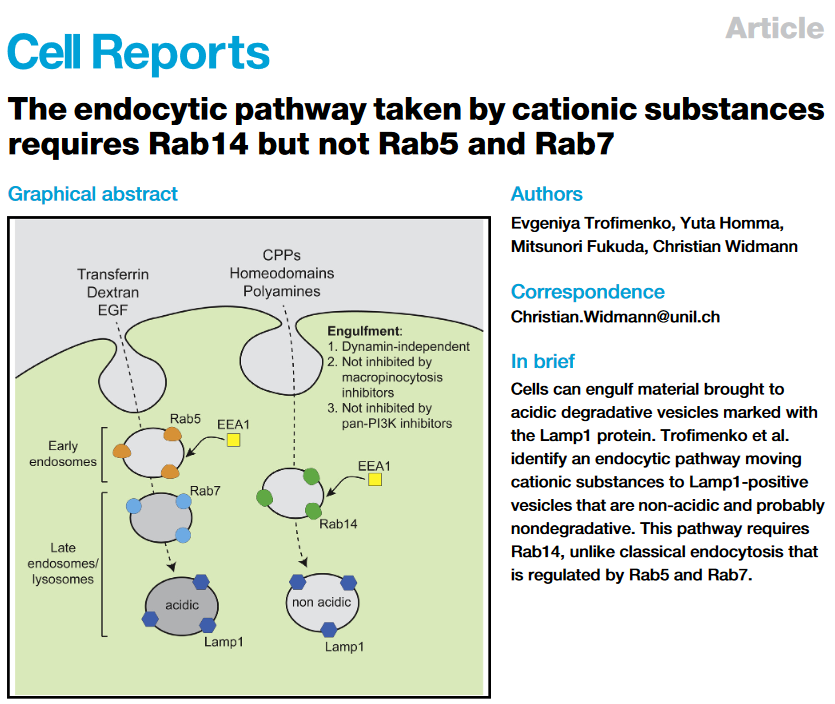
Endocytosis is a major entry route used by cells to take up a variety of extracellular substances. The endocytosed material transits from early endosomes to late endosomes/lysosomes. Even though endocytosis can proceed in a variety of manners, the current evidence indicates that all endocytosed substances follow a single endocytic maturation pathway towards LAMP1-containing lysosomes that is characterized molecularly by the presence of Rab5 on early endosomes and Rab7 on late endosomes. We have discovered that cell-penetrating peptides (CPPs) use a second endosomal pathway en route to non-acidic LAMP1-positive compartment that had escaped detection so far. Progression along this endocytic pathway requires the Rab14 protein but not Rab5 and Rab7. This endocytic route is taken by other, physiological, cationic cargos such as polyamines or homeodomains (HDs) and appear to end up in non-acidic LAMP1-positive compartments that presumably fulfill non-degradative functions. We will now study in more details how the Rab14-dependent pathway operates at the molecular level and investigate its role and function in cells.