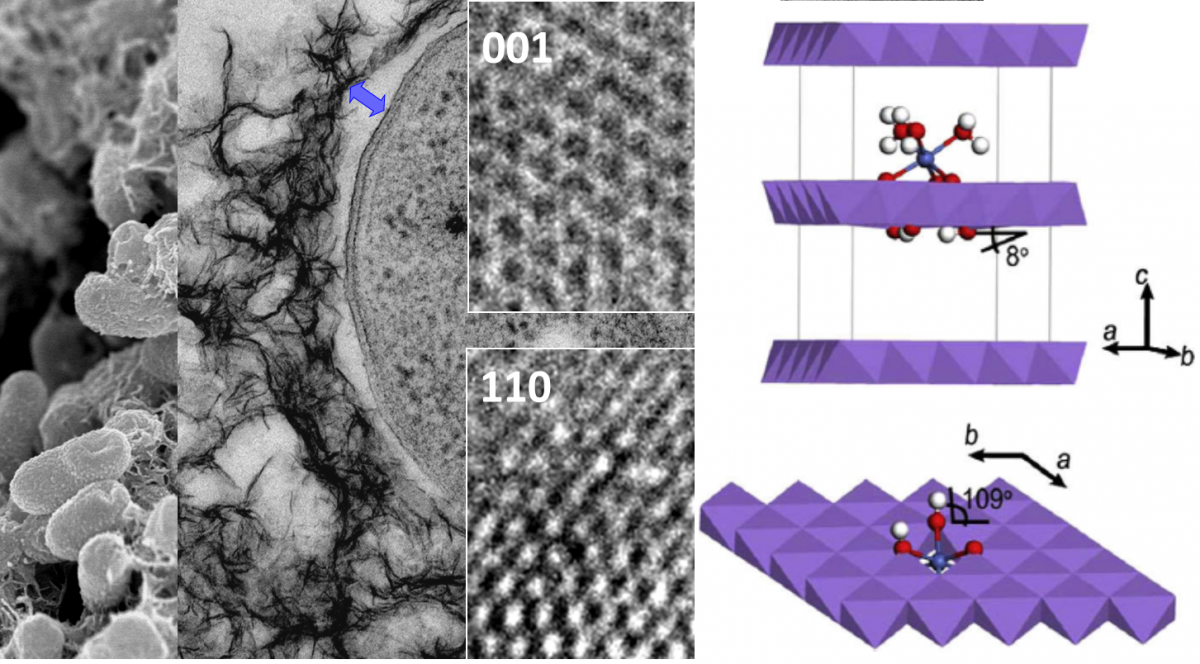
Birnessite minerals (layer-type MnO2) produced by bacteria and fungi participate in important biogeochemical processes in soils, aquifers, and sediments, particularly trace metal sorption. Thus, these minerals highly influence the distribution, mobility and bioavailability of nutrient and contaminant metals in the environment. In addition, birnessite minerals are among the strongest environmental oxidants, contributing to the decomposition of natural organic matter, the oxidative degradation of complex organic pollutants, and the respiration of metal-reducing bacteria in aquatic and terrestrial environments.
Despite their broad importance in many environmental processes, several aspects regarding the reactivity of biogenic birnessite remain poorly understood. Currently, we investigate the sorption reactivity of nanoparticle edges, biofilm-mineral interactions, and (photo)reductive mineral dissolution using a range of biotic and abiotic minerals. We combine the macroscopic-level adsorption and dissolution measurements with molecular-level methods such as X-ray diffraction, Xray absorption spectroscopy (to acquire information about the coordination geometry of adsorbed elements and local structural of host mineral phase), quick X-ray absorption spectroscopy (to acquire time-resolved information on changes in local coordination geometries of elements), infrared spectroscopy (to monitor changes in coordination of the functional groups in biomass) and transmission electron microscopy (to determine particle size, aggregation and crystallinity).
The results from this research have important implications for attenuation of pollution in natural systems, remediation schemes in engineered systems and water treatment; water resource management; and nutrient cycling in terrestrial and marine ecosystems. In addition to providing an improved understanding of processes linked to the Mn biogeochemical cycle, this research has relevance to materials science where Mn(IV) oxides may have important applications related to their electrochemical, photochemical and catalytic properties.