This study investigates how combining artificial intelligence (AI) with traditional retinal image features enhances disease prediction and the discovery of associated genes. By analyzing retinal scans using the deep learning model RETFound and comparing its outputs with clinically measurable traits familiar to physicians, researchers demonstrate that integrating both approaches leads to more accurate health predictions and deeper biological insights.
Combining AI and traditional eye imaging techniques improves disease and gene detection
Artificial intelligence (AI) enables physicians to analyze medical images more efficiently and predict disease risks with greater accuracy. A powerful technique known as deep learning can detect complex patterns in retinal images. However, these systems often produce results that are difficult to interpret, which poses a challenge for their adoption in clinical practice.
In a recent study, a team of researchers examined a deep learning model called RETFound, trained on millions of ocular images. The model’s performance was compared to that of more traditional visual features—such as the shape and size of blood vessels—which are well-established markers in ophthalmology.
The results showed that the internal patterns learned by RETFound did not directly align with individual traditional features. However, combining both approaches led to improved performance. Researchers also trained RETFound to predict these classical traits; while the predictions closely matched measured values, they remained imperfect.
Moreover, the features learned by RETFound were found to be associated with numerous genes, often distinct from those linked to traditional traits. Some of these learned features exhibited stronger genetic influence and proved more effective in predicting certain ocular diseases.
When it comes to broader health issues—such as diabetes, blood pressure, or body weight—the AI-derived features proved highly useful. However, it was the combination of AI and traditional traits that yielded the most accurate predictions.
In summary, this study demonstrates that combining human-interpretable visual features with AI-learned representations enables more accurate assessment of ocular health and related diseases. This integrated approach paves the way for more reliable diagnostics and a deeper understanding of underlying pathological mechanisms.
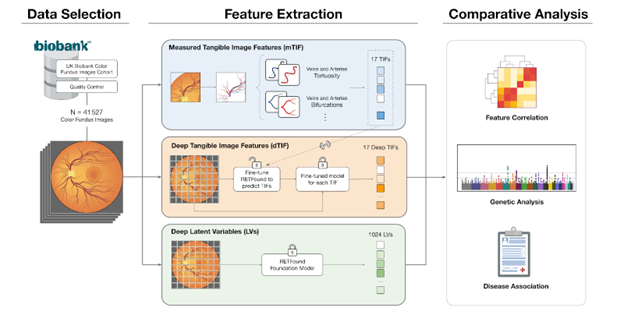
(c) For subsequent comparative analyses, direct correlations, genetic associations, and links to various diseases were investigated.
Preprint: https://doi.org/10.1101/2024.12.23.24319548
Sven Bergman is currently leading the Sinergia project funded by the Swiss National Science Foundation (SNSF), entitled “Linking micro- and macrovascular properties through multimodal imaging, genetics, and deep learning to better understand vascular disease mechanisms and predict health risks.” The project is conducted in collaboration with three medical partners: the Jules-Gonin Eye Hospital, the Inselspital in Bern, and the Erasmus Medical Center in Rotterdam.
The aim of this project is to better understand the interaction between different types of blood vessels, at different scales and in various organs, ranging from very small capillaries to large arteries and veins. Using extensive collections of medical images, the project aims to measure the properties of vessels, link them together, and study their contribution to the risk of cardiovascular disease. To do this, researchers are using machine learning and artificial intelligence tools, which enable them to process large amounts of imaging data and generate prediction tools.
More specifically, the project began with a well-known trait called retinal vessel tortuosity, which describes the “twisting” or curvature of the small arteries and veins that supply the eye’s light-sensitive receptors. This trait has been shown to be strongly influenced by genetics and is closely linked to cardiovascular risk factors such as hypertension. The approach was then expanded to a set of 17 vascular traits—including vessel diameter and density—which were compared to those extracted by RETFound, a foundation model for retinal analysis trained on over one million images, using genetic and disease association tools
Sven Bergmann is a computational biologist designing and applying novel algorithms for the analysis of big biological and medical data.
Faculty of Biology and Medicine
GWAS, Retina, Deep learning, Foundation model, RETFound, Vasculature, CVD
