The purpose of the monitoring activity is to ensure that the rights and welfare of the subjects are protected, and that the clinical trial (ClinO) or clinical trial with medical device (ClinO-MD) data entered into the database is accurate, complete and verifiable from the source documents.
It also oversees the conduct of a clinical trial or a clinical trial with a medical device and ensures that it is conducted, recorded and reported in accordance with the study protocol, applicable quality documents, Good Clinical Practice (GCP) (ICH E6 GCP 5.18) and applicable regulations.
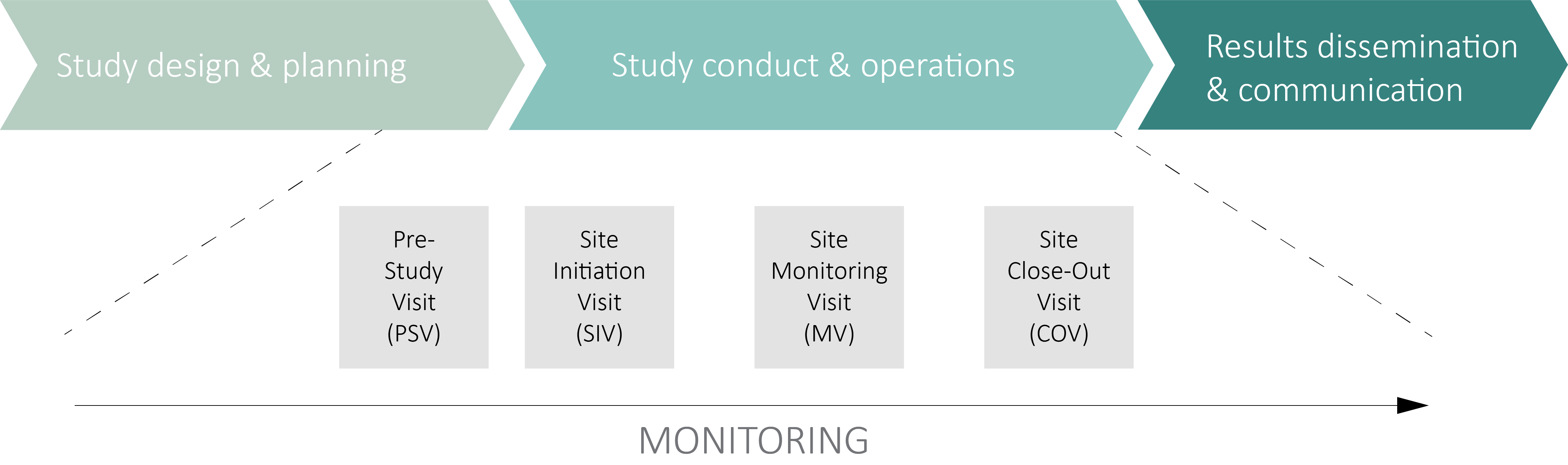
Contact us to find out more about how the monitoring should be set up.

