
1. Contracts, funding and budget
Any agreement in relation to the research project planned or concluded between the sponsor/representative of the sponsor/investigator of the CHUV, and a proxy institution or other third parties must be contractualised. At the CHUV, all contracts such as service contracts, clinical trial contracts, contracts for the exchange of data collected in the framework of research or amendments must be submitted to the administrative head of the department according to the CHUV directive. In addition, all contracts must be reviewed and approved by the CHUV legal department (contrats.afj@chuv.ch) and signed by the director of the CHUV, with the exception of the Confidentiality Disclosure Agreement (CDA) and Data Transfer Agreement (DTA)/Material Transfer Agreement (MTA).
There are several ways to fund your research in Switzerland :
- The Swiss National Science Foundation (SNSF) is Switzerland's leading research funding institution and offers a wide range of research programmes.
- The EU-framework programmes for research and technological development is the most important research instrument in the European Union. Swiss researchers can participate in various projects.
- The Funding support - UNIL, the UNIL research support service.
- Foundations: many Swiss foundations support and finance projects in the field of science and research, including the CHUV Foundation.
It is important to take the time to develop a budget to ensure that there are sufficient funds/budgets to carry out the research project from start to finish. The budget allows for the identification of procedures that are research related and should be billed to the service/research fund/company contract versus standard care billed to the patient's insurance.
What to evaluate to develop a research budget :
- Ensure that the research team has properly assessed effort/activity in hours by trade.
- Ensure that there is a charge for «start-up, close-out & archiving fees», if applicable.
- Consider adding a miscellaneous costs section : reimbursement of patient/volunteer travel, cost of submission to authorities (CER and Swissmedic), protocol amendment, etc.
- Be familiar with the requirements of the CHUV or your institution : overheads, contracts, etc.
If you have any questions or need help, please contact the platform
2. Methodology and statistics
The healthcare research platform helps you to define and carry out the most relevant statistical analyses to answer your research questions.
Study design and statistical analysis are closely linked, which is why it is important to involve statisticians from the design phase of your project. Therefore, do not hesitate to contact the platform as soon as you have a research question.
The research platform helps you calculate the number of participants you need to include (sample size calculation), helps you write the statistical part of the protocol and establish the statistical analysis plan. It also helps you with intermediate analyses (quality control), data cleaning, final analysis and the production of tables and graphs ready for publication.
3. Research data management
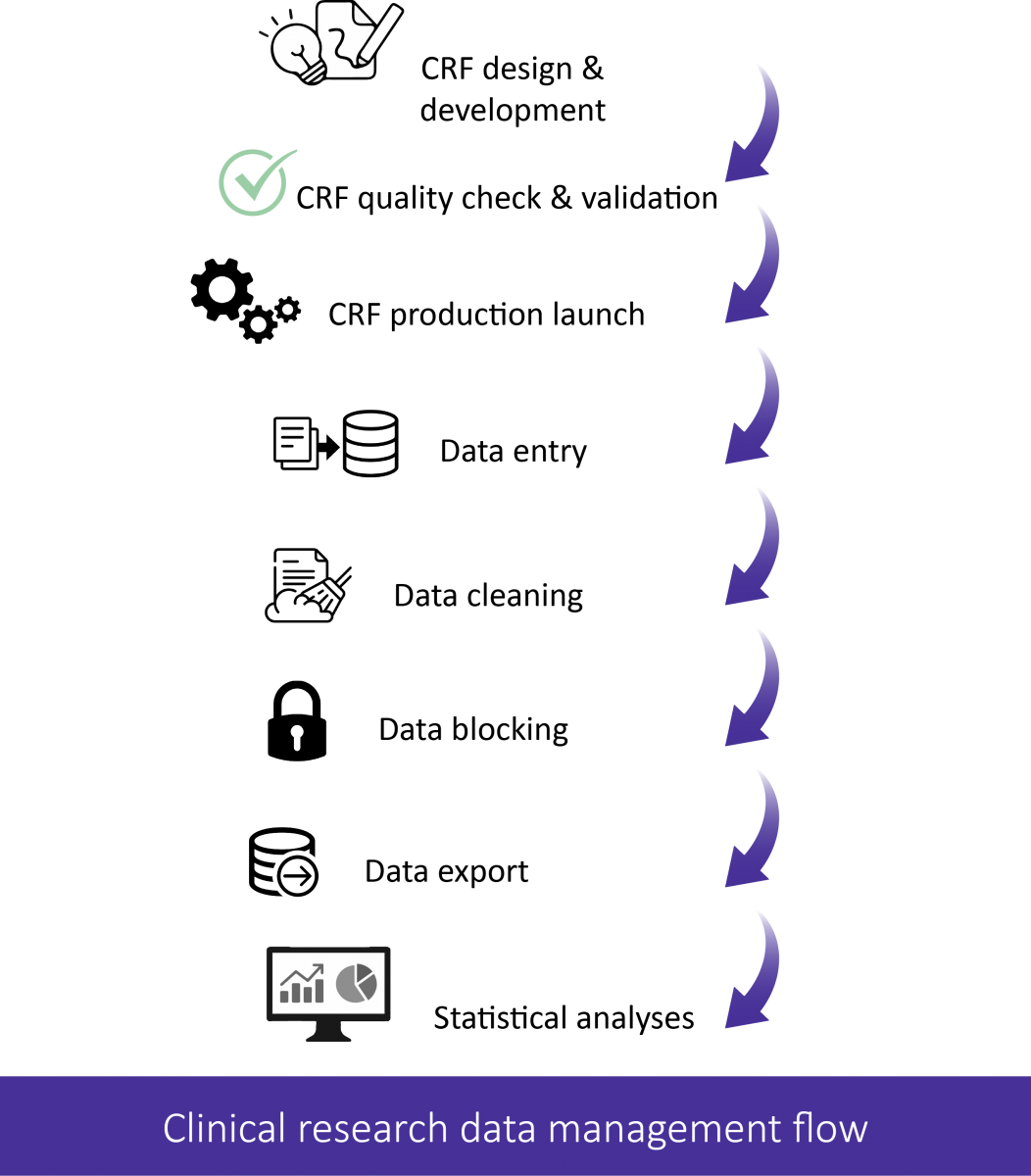
The platform team helps you to define the data management system best suited to your needs and to define the specifications (REDCap, Lime Survey, SPHINX etc.).
The platform can create your project and provide you with access before advising you on the development of your database and performing quality checks. It also has the capacity to develop your database from scratch after defining the specifications.
If you wish to develop a database with REDCap software with structured data entry forms (eCRF: Electronic Case Report Form), the platform can provide you with a dozen REDCap form templates that can be easily adapted to your project. In addition, it can provide support for the different stages of clinical research data management (see diagram above).

