The endodermis: The building of a plant polarized epithelium
The endodermis is the innermost cortical cell layer of plant roots and surrounds their central vascular strand. It features Casparian strips, ring-like hydrophobic cell wall impregnations that surround each endodermal cells as a median belt and which are fused into a supracellular, net-like structure. This Casparian strip network represents the major extracellular (apoplastic) diffusion barrier in young roots. It serves to separate and protect the inner, extracellular space of the vascular cylinder from that of the cortex, which is continuous with the soil. Thereby, the endodermis is functionally equivalent to an animal polarized epithelium, such as the intestinal epithelium, for example. Very little was known in molecular terms about the building of this intricately structured cell layer that has evolved independently from animal epithelia.
In a series of publication in recent years, we were able to describe the progression of endodermal differentiation and obtain molecular markers for three distinct plasma membrane domain an inner and outer domain, separated by a median domain, now termed the “Casparian strip membrane domain” (CSD) (Alassimone et al., PNAS, 2010). We identified a previously uncharacterized family of proteins, now named “CAsparian Strip domain Proteins” (CASPs) as well as “CASP-Likes” (CASPLs) for the extended family of CASP-related proteins (see Roppolo et al., Plant Physiol., 2014). We showed that CASPs are functionally equivalent to animal tight junction proteins, setting up a median domain that acts as a lateral diffusion barrier and organizes the Casparian strip cell wall impregnation itself (see Roppolo et al., Nature, 2011). We clarified a long-standing debate about the molecular nature of this cell wall impregnation, demonstrating that the Casparian strips are made of lignin and not suberin, as often presumed (see Naseer et al., PNAS, 2012). We now propose that CASPs provide a transmembrane protein scaffold that assembles a “lignin polymerizing complex”, consisting of a specific NADPH oxidase, as well as peroxidases. Interestingly, the precisely localized, dense and rapidly forming lignin of the Casparian strip is unique in being absolutely dependent on peroxidases, but not on the equally present laccases (Rojas-Murcia et al., PNAS, 2020).We postulate that many other proteins – such as ESB1, identified by David Salt’s group (Hosmani et al., PNAS, 2013) – as well as currently unidentified proteins are part of this lignin polymerising complex. Our model explains how precise subcellular polymerization of lignin in a centrally located belt might be achieved (Lee et al., Cell, 2012).
In two consecutive forward genetic screens, we identified numerous genes necessary for Casparian strip formation (Alassimone, Fujita et al., Nat Plants, 2016 and Kalmbach et al., Nat Plants, 2017). In our LORD OF THE RINGS (LOTR) screen, we directly scored for mislocalisation of our CASP1-GFP marker. Among the many complementation groups we discovered, lotr2 turned out to be an allele of EXO70A1, a specific exocyst subunit. Interestingly, this mutant does not affect secretion of CASP1-GFP, but leads to a complete mis-localisation of CASP1-GFP into a multitude of small microdomain at the plasma membrane, demonstrating that specific EXO70 subunits in plants are used as localised landmarks for specific secretion processes (Kalmbach et al., Nat Plants, 2017). Another mutant, lotr1, was initially characterised by Toru Fujiwara’s group (Li et al., Current Biology, 2017) and we could recently provide evidence that is encodes a putative extracellular protease that act non-cell autonomously to prevent ectopic formation of CASP1 domains. In the context of this work, we could also demonstrate that CASP domain stability is strictly dependent on CASP domain formation in neighboring cells, through a completely unknown mechanism of cross cell wall communication (Kolbeck, Marhavy et al., eLife, 2022). We have written a number of reviews that summarise and further discuss diverse aspects of our work and of endodermal development in general (see our reviews).
The SCHENGEN pathway:
A versatile system for diffusion barrier quality control
A breakthrough in our understanding of endodermal differentiation came when we realised that four of the five SCHENGEN mutants, identified in our first genetic screen appear to constitute a novel signal transduction pathway which effectively sets up a “barrier surveillance” mechanism ensuring that the supracellular Casparian strip network will be effectively sealed (Doblas et al., Science, 2017; Alassimone, Fujita et al, Nat Plants, 2016, Pfister et al., eLife, 2014). This is achieved by an elegant separation of secreted ligand, produced in the stele, and a receptor signalling module confined to the cortex-facing plasma membrane domain of the endodermis. Such a setup ensures that signalling will only cease once any discontinuity in the Casparian strip has been sealed (Doblas et al., Science, 2017). Recently, we could demonstrate that the molecular outlines of the SCHENGEN pathway resembles plant pattern-recognition pathways. Yet, the specific subcellular localization of its signaling components is central to its functionality, with a plasma membrane branch of the pathways allowing for very direct and localised induction of lignification and a cytoplasmic branch, leading to upregulation of many lignification, suberization, as well as endodermal differentiation genes (Fujita et al., EMBOJ, 2020). We also contributed to work from Michael Hothorn’s lab, that solved the structure of the very big LRR ectodomain of the SGN3 (GSO1) receptor and identified additional ligands for SGN3 (Okuda et al., PNAS, 2020), as well as work from Gwyneth Ingram’s lab, that demonstrated an intriguing variation on the SCHENGEN pathway that is crucial for diffusion barrier surveillance during embryo development, promoting formation of a functional embryonic cuticle (Doll et al., Science, 2020).
The functional relevance of the endodermis
Our markers and specific mutants in endodermal barrier formation have become invaluable tools to understand the many supposed roles of the endodermis in root function, specifically its role in the selective uptake and retention of nutrients, but also its proposed function as a protective barrier to pathogens, for example. Using the sgn3 mutant for example, we could demonstrate that its severely disrupted Casparian strips are causing complex changes in its leaf ionomic profile, among which, a very robust reduction in potassium levels. This appears to be largely responsible for the overall growth reduction and low potassium hypersensitivity of the mutant (Pfister et al., eLife, 2014).
More recently, we have focused more attention on endodermal suberisation, a well described “secondary state” of endodermal differentiation in which cells surround themselves with a coat of hydrophobic suberin. Suberisation is bound to have large impact on the ability of endodermal cells to sense biotic and abiotic stresses and to take up nutrients. Indeed, we could show that a number of distinct nutrient stresses either enhance or decrease endodermal suberisation, through increases in abscisic acid (ABA) or ethylene hormone, respectively. These changes in suberisation are physiologically adaptive in that they can provide for increased uptake or retention of a given element (Barberon et al, Cell, 2017 ). Intriguingly, even within a fully suberized endodermis, some cells are kept in an unsuberised state. Although described for a long time, the development and function of these so-called “passage cells” has remained obscure. We could demonstrate that passage cells arise from a previously undetected bisymmetry in the endodermis, which can be subdivided into smaller, less cytokinin- and ABA-sensitive, xylem-pole endodermis and longer, more cytokinin- and ABA-sensitive phloem-pole endodermis. This difference arises in part from movement of a stele-expressed cytokinin repressor protein into the endodermis (Andersen et al., Nature, 2018 ). Moreover, phosphate transport genes are expressed specifically in passage cells and the presence of passage cells even appears to influence expression of transport genes in surrounding cortex and epidermal cells.
A number of years ago, we could also demonstrate the crucial importance of endodermal responses for the accommodation of early lateral root formation, a process that requires dramatic morphological and physiological changes by the neighboring endodermal cells in order to allow growth and eventual passage of a lateral root primordium across the endodermal cell layer (Vermeer et al., Science, 2014). In collaboration with Joop Vermeer’s lab, we could recently demonstrate that one aspect of endodermal accommodation involves de-suberisation of the endodermis and identified a number of GDSL lipases that can drive de-suberisation in the endodermis. In this context, we also demonstrated that different types of GDSL lipases are collectively required for suberin formationa and very probably represent the hitherto unknown enzymes that mediate suberin polymerization in the cell wall (Ursache et al., Nature Plants, 2021).
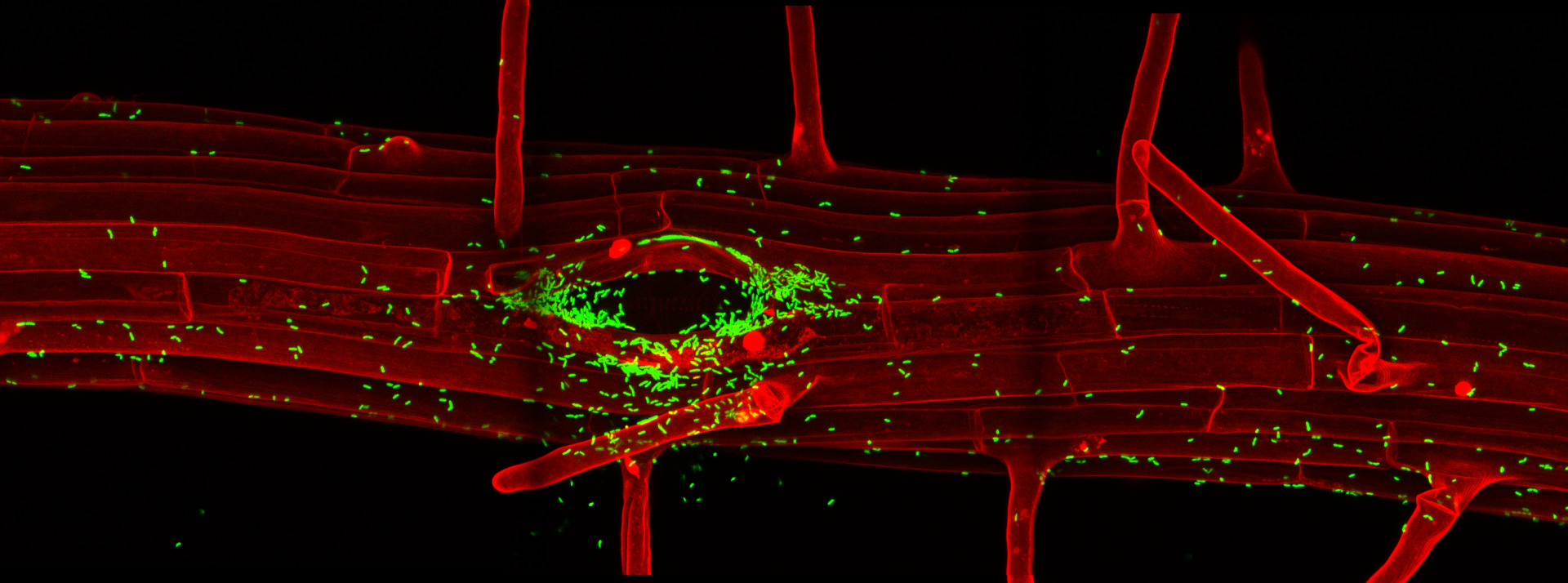
The specificities of root immune responses
In a collaborative efforts with the labs of Thomas Boller and Jean-Pierre Métraux, we have developed a number of fluorescent marker lines that would allow us to map root stress and immune responses at cellular resolution (Poncini et al., PLOS One, 2017). Using these lines in combination with a single cell laser ablation setup, we were able to demonstrate that single cell damage in differentiated roots causes a trio of regional responses consisting of surface depolarization, ROS production and induction of a Calcium wave. Surprisingly, we found that this cellular damage casues a regional induction of ethylene production and signaling, but does not induce a jasmonate response. We could further demonstrate that this “ethylene-only” damage response is nonetheless protective against nematode invasion, which causes similar regional ethylene responses (Marhavy et al., EMBOJ, 2019). Importantly, by using our cellular resolution transcriptional response markers for microbial pattern signaling, we could demonstrate that differentiated plant roots are very insensitive to the presence of microbial, especially bacterial patterns, but become powerfully “gated” by local cellular damage. We demonstrate that presence of cellular damage can also cause an immune response to otherwise tolerated commensal bacterial colonizers. This has led us to propose a co-incidence model in which roots are rather insensitive to the mere presence of bacterial molecular patterns in the absence of cellular damage – thus allowing for bacterial colonization of benign commensal bacteria – while mounting defenses against potentially pathogenic bacteria that cause cellular damage (Zhou et al., Cell, 2020). We also recently demonstrated the importance of spatially restricting immune responses in roots by installing expression of the FLS2 pattern recognition receptor in root meristematic epidermal cells. The consequent, flg22 peptide-triggered, strong immune response in those cells causes rapid meristem disfunction, associated with extensive lignification. In some cases, this also causes root growth inhibition in response to commensal bacteria that are otherwise tolerated by wild-type roots (Emonet et al., Current Biology, 2021).