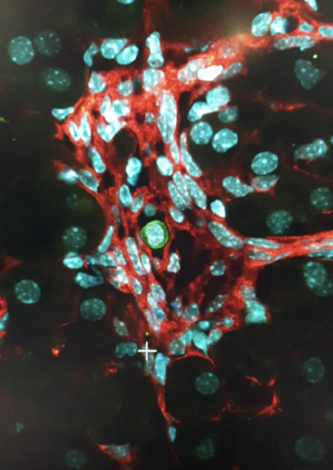
The Laboratory of Regenerative Hematopoiesis strives for a better understanding of blood cell formation and, in particular, of the hematopoietic microenvironment within the bone marrow or in other adult sites amenable to host functional hematopoietic stem cell proliferation.
Inducible CXCL12/CXCR4-dependent extramedullary hematopoietic niches in the adrenal gland
Adult hematopoietic Stem and Progenitor Cells (HSPCs) reside in the bone marrow hematopoietic niche, which regulates HSPC quiescence, self-renewal, and commitment in a demand-adapted manner. While the complex bone marrow niche is responsible for adult hematopoiesis, evidence exists for simpler, albeit functional and more accessible, extramedullary hematopoietic niches. Inspired by the anecdotal description of retroperitoneal hematopoietic masses occurring at higher frequency upon hormonal dysregulation within the adrenal gland, we hypothesized that the adult adrenal gland could be induced into a hematopoietic supportive environment in a systematic manner, thus revealing mechanisms underlying de novo niche formation in the adult. We have been able to show that upon splenectomy and hormonal stimulation, the adult adrenal gland of mice can be induced to recruit and host functional HSPCs, capable of serial transplantation, and that this phenomenon is associated with de novo formation of platelet-derived growth factor receptor α (PDGFRα) expressing stromal nodules. We further show in CXCL12-GFP reporter mice that adrenal glands contain a stromal population reminiscent of the CXCL12-Abundant Reticular (CAR) cells which compose the bone marrow HSPC niche. Mechanistically, HSPC homing to hormonally-induced adrenal glands was found dependent on the CXCR4/CXCL12 axis. Mirroring our findings in mice, we found reticular CXCL12+ cells co-expressing master niche-regulator FOXC1 in primary samples from human adrenal myelolipomas, a benign tumor composed of adipose and hematopoietic tissue. Our findings reignite long-standing questions regarding hormonal regulation of hematopoiesis and provide a novel model to facilitate the study of adult-specific inducible hematopoietic niches which may pave the way to therapeutic applications.
In vitro interrogation of the Human Bone Marrow Adipogenic Axis in the context of hematopoiesis
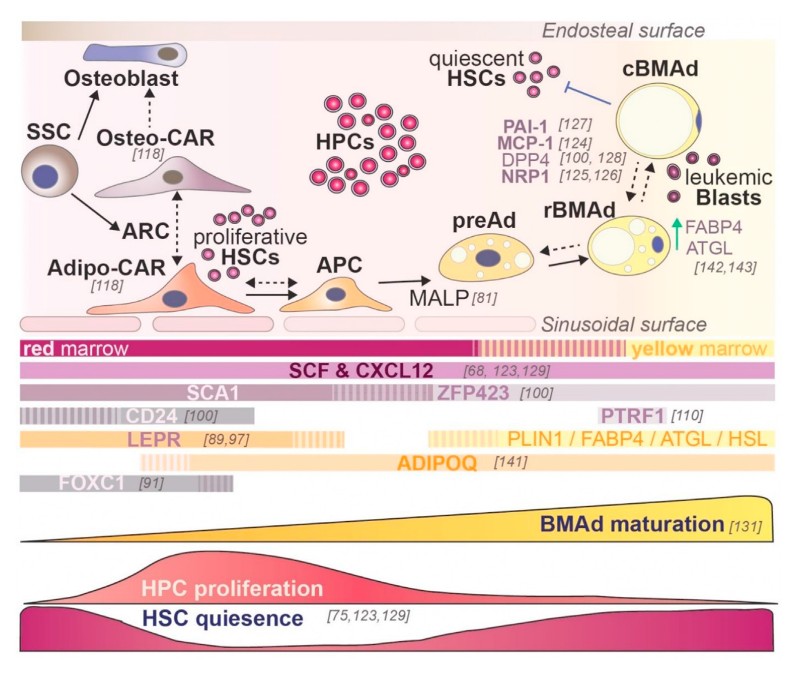
TThe bone marrow (BM) microenvironment consists of a complex network of different cell populations working collectively to orchestrate the regulation of hematopoiesis. Understanding the cellular and functional heterogeneity of the BM stromal compartment is imperative in order to unravel targets and factors that may be modulated by clinical interventions for hematological disorders. Among the stromal components, the constituents of the BM adipogenic differentiation axis are of particular interest, as it was observed in mice models that BM adipocytes can both positively and negatively influence hematopoiesis in a context-dependent manner, and several subpopulations (AdipoCARs and MALPs) identified recently through scRNAseq experiments have been shown to be critical components of the hematopoietic niche. This axis in the context of the human BM, has been examined less extensively. We aim to show that different cell states within the adipogenic differentiation stages of human BM stromal cells (hBMSCs) contribute differently to hematopoiesis. Primary and immortalized hBMSC cell lines induced to undergo different levels of adipogenic differentiation are used in in vitro co-culture systems together with primary human hematopoietic and progenitor cells.