
POSTDOCTORAL POSITION: DeepMarrow collaborative TANDEM project
Introduction
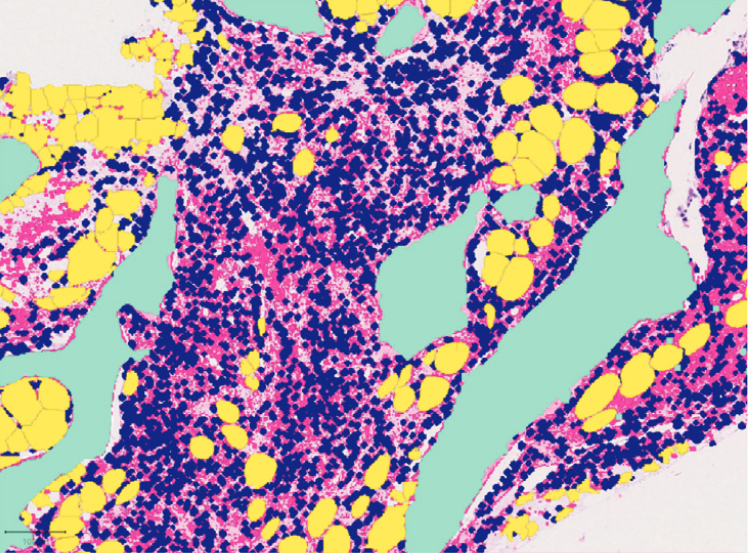
A newly funded ISREC Foundation TANDEM project is in search of a highly motivated postdoc to bring together clinicians, engineers and computational biologists to develop, train and apply an artificial intelligence-based digital histopathology workflow named DeepMarrow to contribute to outcome stratification in acute myeloid leukemia (AML). The team is based in Lausanne, Switzerland, and encompasses three institutions : (i) the University of Lausanne (UNIL) Faculty of Biology and Medicine, a leading public institution of higher education and research in Switzerland, (ii) the Lausanne University Hospital, repeatedly ranked as one of the 20 best academic hospitals in the world, and (iii) the École Polytechnique Fédérale de Lausanne, one of the world’s most prestigious engineering, technology and science institutions. The collaborating laboratories are part of the Swiss Cancer Center Leman network (https://sccl.ch/).
Presentation
A postdoctoral position is available at the UNIL-CHUV Laboratory of Regenerative Hematopoiesis, coled by Prof. Olaia Naveiras (Department of Biomedical Sciences https://wp.unil.ch/naveiras-lab/team/ and Hematology Service Translational Research Unit https://www.chuv.ch/en/hematologie/hemhome/ research/) and by Dr. Daniel Sage at the EPFL Biomedical Imaging Group (https://bigwww.epfl.ch/). The project focuses on the study of unexplored morphological features and spatial relationships in bone marrow tissue sections from patients undergoing induction chemotherapy for AML, with benchmarking against other scenarios of intense bone marrow remodeling. Key contributing collaborators to the project are Prof. Laurence de Leval (Institute of Pathology, CHUVUNIL), Prof. Bart Deplancke (Institute of Bio-engineering, EPFL) and Prof. Sabine Blum (Service of Hematology, CHUV).
Job information
- Expected start date in position: 01.03.2025 or subject to work visa approval
- Contract duration: 24 months (12+12 months, with possibility of a 3rd year)
- Percentage of activity: 80-100%
- Location: Lausanne, UNIL-FBM (CHUV Bugnon campus)
Your responsibilities
We are looking for a rigorous, creative, and team-driven individual with a passion for and ideally already substantial expertise in image analysis and related machine learning approaches and a desire for clinical impact. The selected candidate will (i) collaborate closely with clinicians to securely structure the longitudinal bone marrow image cohort and coded clinical data associated to the three currently approved local, national and international cohorts, while (ii) developing the DeepMarrow workflow and morphological feature extraction from the available training set (doi:10.1016/j.modpat.2022.100088) and (iii) identifying specific samples for multimodal discovery analysis (multiplex immunohistochemistry and spatial transcriptomics). Guaranteed in-depth exposure to machine learning, advanced hematopathology, multimodal omics analyses including spatial transcriptomics and marrow stromal niche biology within a highly motivated and collaborative team.
Your qualifications
- PhD or MD/PhD degree in computational biology or pathology, preferably in the fields of cancer or stem cell biology
- Highly autonomous in computational analysis, skilled in image and/or transcriptomic data processing, and able to manage projects that integrate data curation and deep learning workflows.
- Wet-lab expertise in cell biology or experimental pathology (e.g. morphological tissue assessment, H&E, immunohistochemistry, primary human tissue processing) is a must.
- Prior experience in obtaining ethical approval and handling of human primary tissues, including knowledge of the legal and ethical framework (e.g. TRREE certification) will be highly valued
- Expertise in diagnostic hematopathology or experimental hematology will be highly valued
- Demonstrated capacity for project management in a highly collaborative setting is a must
- Fluency in English with outstanding oral and written communication skills.
Preference will be given to outstanding candidates with a solid record of peer-reviewed publications and/or selective international conference presentations available to start in March-April 2026.
Contact for further information
Prof. Olaia Naveiras and Dr. Daniel Sage
Email (all three please): olaia.naveiras@unil.ch, daniel.sage@epfl.ch, tatiana.smirnova.1@unil.ch
Subject line : “TANDEM DeepMarrow position”
What the position offers you
A dynamic, stimulating, interdisciplinary research environment, state-of-the-art technologies, cuttingedge core facilities, and attractive employment conditions in addition to fascinating life in Switzerland. The UNIL/CHUV medical campus and the EPFL campus are in Lausanne, in close proximity from Lake Geneva and the Swiss Alps.
Your application
Applications should include a motivation letter, complete CV and at least two reference letters (including a letter from the PhD advisor, unless duly justified). Selected candidates will be contacted by mid January for interviews on Thursday January 22nd.
Please send your full application in PDF format.
Deadline for applications: January 2nd 2026
Selected candidates will be contacted and invited for interviews on January 2026. Only applications addressed to all three emails and with the specified subject line will be considered.
We thank you for your understanding.
Additional information
UNIL is committed to promoting gender equality and strongly encourages applications from female candidates.
MASTER’S THESIS POSITION:
Separation of primary cell adipocytes by Deterministic Lateral Displacement
This master thesis project focuses on the development of a novel Deterministic Lateral Displacement (DLD) platform tailored for sorting primary adipocytes obtained from human tissue biopsies. While DLD systems have demonstrated great potential in sorting various blood cell types, their application to isolating primary cells from tissue digestion remains largely unexplored. The goal of this project is to build upon a DLD device previously developed in our laboratory and expand its utility toward the efficient isolation of bone marrow adipocytes.
The thesis will involve redesigning and optimizing the existing DLD platform to accommodate the unique characteristics of primary adipocyte samples. Key objectives include adjusting the critical sorting size, modifying microfluidic geometries to manage heterogeneous cell populations, and implementing strategies to prevent chip clogging. A significant part of the project will also focus on optimizing the biological pre-processing of the tissue biopsy. This includes refining the digestion protocol to preserve fragile adipocytes, removing lipid contamination, and achieving a monophasic cell suspension suitable for microfluidic sorting. The student will work closely with both the Laboratory of Life Sciences Electronics (CLSE-EPFL) and the Laboratory of Regenerative Hematopoiesis (Naveiras group-UNIL) to iteratively test and improve both the biological and engineering aspects of the workflow.
Type of work: 10% literature study, 20% design and microfabrication, 25% cell culture and biological characterization, 25% microfluidic device testing, 20% data treatment and results reporting
Duration: 6 months
Prerequisites: Ideally, someone with a background in bioengineering, biology or chemistry, but physics or microtechnology are also welcome. A strong motivation is required regardless of your background.
Interested candidates are encouraged to contact us via email and include their CV. While a reference letter is not required, it would be considered an asset: micaela.cristofori@epfl.ch, fabien.bonini@unil.ch
MASTER’S THESIS POSITION:
Feeling the pressure: the role of Piezo1 during red-to-yellow transition in aplastic bone marrow
Introduction
Piezo1 is a mechanosensitive, nonselective cation channel that transduces physical forces into intracellular calcium signals1. Piezo1 enables cells to sense and respond to mechanical cues such as matrix stiffness and cellular crowding2. It is expressed in multiple cell types, including bone marrow resident cells such as hematopoietic stem and progenitor cells (HSPCs) and adipocyte lineage cells. In the latter, Piezo1 acts as a key regulator of adipogenic differentiation: its activation is associated with reduced adipocyte differentiation, whereas downregulation of Piezo1 promotes adipogenic commitment3.
While the contribution of Piezo1 to bone health is increasingly well defined, its role in pathological remodelling of the marrow niche remains poorly understood. For instance, in chemotherapy-induced bone marrow aplasia, there is a rapid and profound decline in marrow cellularity, characterized by severe depletion of hematopoietic (red) marrow and its progressive replacement by adipocyte-rich (yellow) marrow. Given the known function of Piezo1 as a mechanosensor and its inhibitory/promoting role in adipogenic differentiation, alterations in Piezo1 signalling may represent a key mechanism linking biomechanical changes of the bone marrow such as decreased cellularity and the abnormal formation of adipocytes during marrow aplasia.
Aim of the project
The aim of the project is to investigate the role of Piezo1 in the red-to-yellow transition of aplastic marrow tissue in a bone marrow adipocyte (BMAd) lineage-specific Piezo1 knockout mouse model (Osterix-FLPo;FLPo-dependentAdipoq-Cre;Piezo1-/- as described in4, nicknamed BMAd-Piezo1-/-) as well as using conventional and advanced in vitro models.
Experimental approaches
For this Master project, the successful candidate should be motivated to work with animals and must obtain a valid RESAL/FELASA Module 1 certification. The candidate will first characterize the BMAd-Piezo1-/- by comparing the bone marrow tissue architecture, cellular density and composition using microscopy and flow cytometry techniques under physiological conditions as well as upon radiation-induced aplastic marrow. Furthermore, the biomechanical properties (e.g. stiffness or elasticity) of the bone marrow tissue including will be investigated using a nanoindenter according to methods already established in our lab. In parallel, she/he will perform 2D/3D ex vivo coculture of stroma and stroma-derived adipocytes from BMAd-Piezo1-/- and control mice with HSPCs, in standard cell culture well-plates or microfluidic devices. Finally, the candidate will conduct quantitative image analysis (ImageJ or QuPath) and flow cytometry analysis using FlowJo as well as process the resulting datasets using statistical software such as GraphPad Prism and 3D design with CAD software (i.e Fusion360) for minor microfluidic device adjustments.
Significance
This work offers a new perspective on the highly plastic bone marrow red-to-yellow transitions5 by exploring a biomechanically-centered mechanism. Its significance lies in identifying Piezo1-mediated mechanosensing as a potential missing link between chemotherapy-induced profound remodelling of the bone marrow microenvironment and the abnormal formation of BM adipocytes which is thought to prime cellular proliferation upon recovery post-chemotherapy in blood cancer. Ultimately, this study may contribute to improving our understanding of how to preserve or restore a hematopoietic-supportive microenvironment by targeting Piezo1-dependent pathways.
Please note that this master project is restricted to UNIL master students. Interested candidates are encouraged to contact us via email: fabien.bonini@unil.ch and olaia.naveiras@unil.ch.
References
- Coste B, Mathur J, Schmidt M, et al. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science. 2010;330(6000):55-60. doi:10.1126/science.1193270
2. Gudipaty SA, Lindblom J, Loftus PD, et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543(7643):118-121. doi:10.1038/nature21407
3. Wang B, Liu J, Wang Q, et al. Piezo1 activation suppresses bone marrow adipogenesis to prevent osteoporosis by inhibiting a mechanoinflammatory autocrine loop. Signal Transduct Target Ther. 2025;10(1):357. doi:10.1038/s41392-025-02455-w
4. Li Z, Bagchi DP, Zhu J, Bowers E, Yu H, Hardij J, Mori H, Granger K, Skjaerlund J, Mandair G, Abrishami S, Singer K, Hankenson KD, Rosen CJ, MacDougald OA. Constitutive bone marrow adipocytes suppress local bone formation. JCI Insight. 2022 Nov 8;7(21):e160915. doi: 10.1172/jci.insight.160915.
5. Tratwal J, Rojas-Sutterlin S, Bataclan C, Blum S, Naveiras O. Bone marrow adiposity and the hematopoietic niche: A historical perspective of reciprocity, heterogeneity, and lineage commitment. Best Pract Res Clin Endocrinol Metab. 2021 Jul;35(4):101564. doi: 10.1016/j.beem.2021.101564. Epub 2021 Aug 10. PMID: 34417114.
MASTER’S THESIS POSITION:
MarrowPatch: an injectable and extramedullary hematopoietic cell delivery system for supportive care in hematology
Introduction
In line with recent advancements in regenerative medicine and novel understanding of naturally occurring ectopic hematopoietic niches [Ref. 1] the Laboratory of Regenerative Haematopoiesis has developed an ectopic bone marrow artificial niche (eBMAN) system to decipher the role of the bone marrow (BM) microenvironment in hematopoiesis while also serving as an in situ blood cell factory [Ref. 2]. This advanced cell therapy combines BM stromal cells and hematopoietic stem and progenitor cells (HSPCs), seeded into a porous biomaterial and implanted in a minimally invasive fashion in the subcutaneous space [Ref. 3-5].
Aim of the project
The ectopic nature of the host tissue presents several challenges, including the need to rapidly induce the de novo formation of a dense and functional vascular network to ensure the survival and controlled expansion of transplanted cells over clinically meaningful time periods. The aim of this Master project is therefore to deepen our understanding of the vascularization processes that support these niches. Establishing a fully functional ectopic BM environment requires not only the formation of a well-organized vascular network capable of sustaining a highly cellular hematopoietic tissue but also the development of specialized vessels (also known as fenestrated sinusoid capillaries) that enable the efficient egress of mature blood cells and platelets into the host circulation.
Experimental approaches
For this Master project, the successful candidate should be motivated to work with animals and must obtain a RESAL/FELASA Module 1 certification. She/he will begin with an extensive literature review on fenestrated sinusoid capillaries to identify specific and reliable markers. She/he will work with the well‑established and extensively characterized immunodeficient NSG mouse model, which will serve as the recipient for eBMAN containing human primary stromal cells and hematopoietic stem cells. After several weeks in vivo, the candidate will perform organ harvesting and sample processing for analysis by flow cytometry and histology including both conventional fluorescent microscopy and electron microscopy (currently the only technique to identify fenestrated sinusoid capillaries). In parallel, the student will be trained to isolate circulating CD34⁺ cells from human buffy coats under P2. The project will further provide hands‑on experience with 2D and 3D human cell culture systems, biomaterial preparation, histology workflows, and flow cytometry-based characterization. Finally, the candidate will conduct quantitative image analysis (ImageJ or QuPath) and flow cytometry analysis using FlowJo as well as process the resulting datasets using statistical software such as GraphPad Prism.
Significance
Vascularization is a fundamental component of all tissues. In the bone marrow niche, in particular, the highly specialized vascular architecture and its remarkable density are essential for maintaining tissue homeostasis, enabling the egress of mature blood cells, and even contributing to platelet maturation. Therefore, a critical next step in the development of our eBMAN system is to recapitulate such a specialized vascular network in an extramedullary environment. Ultimately, eBMAN has the potential to transform traditional transfusion medicine, especially in the context of supportive care for cancer medicine, while advancing our understanding of the complex microenvironment of the BM. For the candidate, it is an opportunity to get experience on biomaterials, artificial niches and hands-on exposure on the whole computer-assisted quantitative histology workflow.
Please note that this master project is restricted to UNIL master students. Interested candidates are encouraged to contact us via email: fabien.bonini@unil.ch and olaia.naveiras@unil.ch.
References
[1] Schyrr F, Alonso-Calleja A, Vijaykumar A, Sordet-Dessimoz J, Gebhard S, Sarkis R, Bataclan C, Ferreira Lopes S, Oggier A, de Leval L, Nombela-Arrieta C, Naveiras O. Inducible CXCL12/CXCR4-dependent extramedullary hematopoietic niches in the adrenal gland. Blood. 2024
[2] Tavakol DN, Tratwal J, Bonini F, Genta M, Campos V, Burch P, Hoehnel S, Béduer A, Alessandrini M, Naveiras O§, Braschler T§. Injectable, scalable 3D tissue-engineered model of marrow hematopoiesis. Biomaterials. 2020
[3] Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019 May;20(5):303-320. doi: 10.1038/s41580-019-0103-9. PMID: 30745579; PMCID: PMC6483843.
[4] Chen J, Hendriks M, Chatzis A, Ramasamy SK, Kusumbe AP. Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. J Bone Miner Res. 2020 Nov;35(11):2103-2120. doi: 10.1002/jbmr.4171.
[5] Bessy T, Itkin T, Passaro D. Bioengineering the Bone Marrow Vascular Niche. Front Cell Dev Biol. 2021 Apr 28;9:645496. doi: 10.3389/fcell.2021.645496. PMID: 33996805; PMCID: PMC8113773.
MASTER’S THESIS POSITION:
How does aging associate to frailty? Dissecting longitudinal changes in immune cell populations and transcriptional patterns during immune-aging.fection and their association to functional decline
Introduction
Aging is a complex biological process characterized by a functional decline that limits lifespan and increases morbidity in most living organisms. Aging also constitutes a major risk factor for most diseases and a socio-economical challenge for modern societies. Immunosenescence is a progressive aging-related process that results in the dysregulation and dysfunction of the innate and adaptive immune system1-2. Consequently, older adults show a decline in immune function leading to higher susceptibility to infectious diseases which is assumed as unavoidable with old age.
Traditionally, studies of immune responses to viral infection have focused on the primary infection site and secondary lymphoid organs such as the spleen3-7. A systematic characterization of how aging affects immune responses across hematopoietic and multiple lymphoid organs has been lacking. To address this, our laboratory has recently generated a comprehensive atlas during a respiratory viral infection describing age-related immune alterations in key tissues, including the primary infection site (lungs), spleen, and bone marrow. This atlas revealed significant differences in the distribution and function of immune populations in older mice compared to young controls. Building on these findings, the next critical step is to validate the identity the altered immune subsets and associated transcriptional patterns using complementary experimental approaches.
Aim of the project
The aim of this project is to contribute to the validation of age-associated immune alterations, which have been preliminarily identified in our scRNAseq transcriptomic atlas of the aged response during viral infection. Once changes in the candidate populations have been quantitatively validated through flow cytometry , we will confirm associated transcriptional signatures and correlation with protein expression and localization using immunohistochemistry (IHC), immunofluorescence (IF) and histology. The observed alterations in transcription factor expression patterns will require validation at the mRNA and protein level. Additionally, the student will have the opportunity to begin investigating potential approaches to mitigate the impact of transcription factors in aged bone marrow. This may involve pharmacological or genetic strategies, both in vitro and in vivo, aimed at reducing the expression of transcription factors or modulating their downstream signalling pathways to restore immune homeostasis.
Experimental approaches
The candidate will begin by learning how to perform flow cytometry data analysis with FlowJo and how to perform immunohistochemistry (IHC), including sample preparation, staining, image acquisition, and data analysis. In parallel, the candidate will contribute to the development and comparison of the histological and immunofluorescence pipeline for the analysis of multiple organs at different time points following viral infection using several markers for immune populations or transcriptional factors (i.e.CD45, CD3, CD4, CD8, CD19, CD11b, CD11c, Gr1, CD117, Tbet, Foxp3). This will involve optimizing tissue embedding, sectioning, and staining protocols, as well as image acquisition and digital analysis. Together, these approaches will allow for the validation of immune alterations identified in the atlas and enable spatial and temporal characterization of affected immune populations across organs. For this Master’s project, the successful candidate should be motivated to work with animals, and be willing to obtain a RESAL/FELASA Module 1 certification depending on FBM rules on eligibility.
Significance
Validation of age-associated immune alterations across lymphoid organs is essential to confirm the robustness of the atlas and to strengthen its value as a reference for studying immune aging. By combining histology and immunohistochemistry, this project will provide spatial and quantitative confirmation of affected immune populations during viral infection. For the candidate, this project offers hands-on training in core immunostaining techniques and data analysis, providing a strong foundation for independent research.
Please note that this master project is restricted to UNIL master students. This project will be co-directed by Prof. Naveiras and Dr. Desdin-Mico (Junior group leader). Interested candidates are encouraged to contact us via email: gabriela.desdin@unil.ch and olaia.naveiras@unil.ch.
References
- Goronzy, J. J. & Weyand, C. M. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 14, 428–436 (2013).
- Desdín-Micó, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
- Williams-Bey, Y., Jiang, J. & Murasko, D. M. Expansion of regulatory T cells in aged mice following influenza infection. Mech Ageing Dev 132, 163–70 (2011).
- Beli, E. et al. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech Ageing Dev 132, 503–10 (2011).
- Jiang, J., Fisher, E. M. & Murasko, D. M. Intrinsic defects in CD8 T cells with aging contribute to impaired primary antiviral responses. Exp Gerontol 48, 579–86 (2013).
- Parks, O. B. et al. Terminally exhausted CD8+ T cells contribute to age-dependent severity of respiratory virus infection. Immun Ageing 20, 40 (2023).
- Torrance, B. L. et al. Senolytic treatment with dasatinib and quercetin does not improve overall influenza responses in aged mice. Frontiers in Aging 4, (2023).