Does my project fall within the scope of the Human Research Act (HRA) or is it outside the HRA?
This law aims to protect the dignity, la personality and health of the human being (Art. 1 HRA)

If my research project falls within the scope of the HRA, a submission to the ethics committee is required, so which ordinance should I refer to?
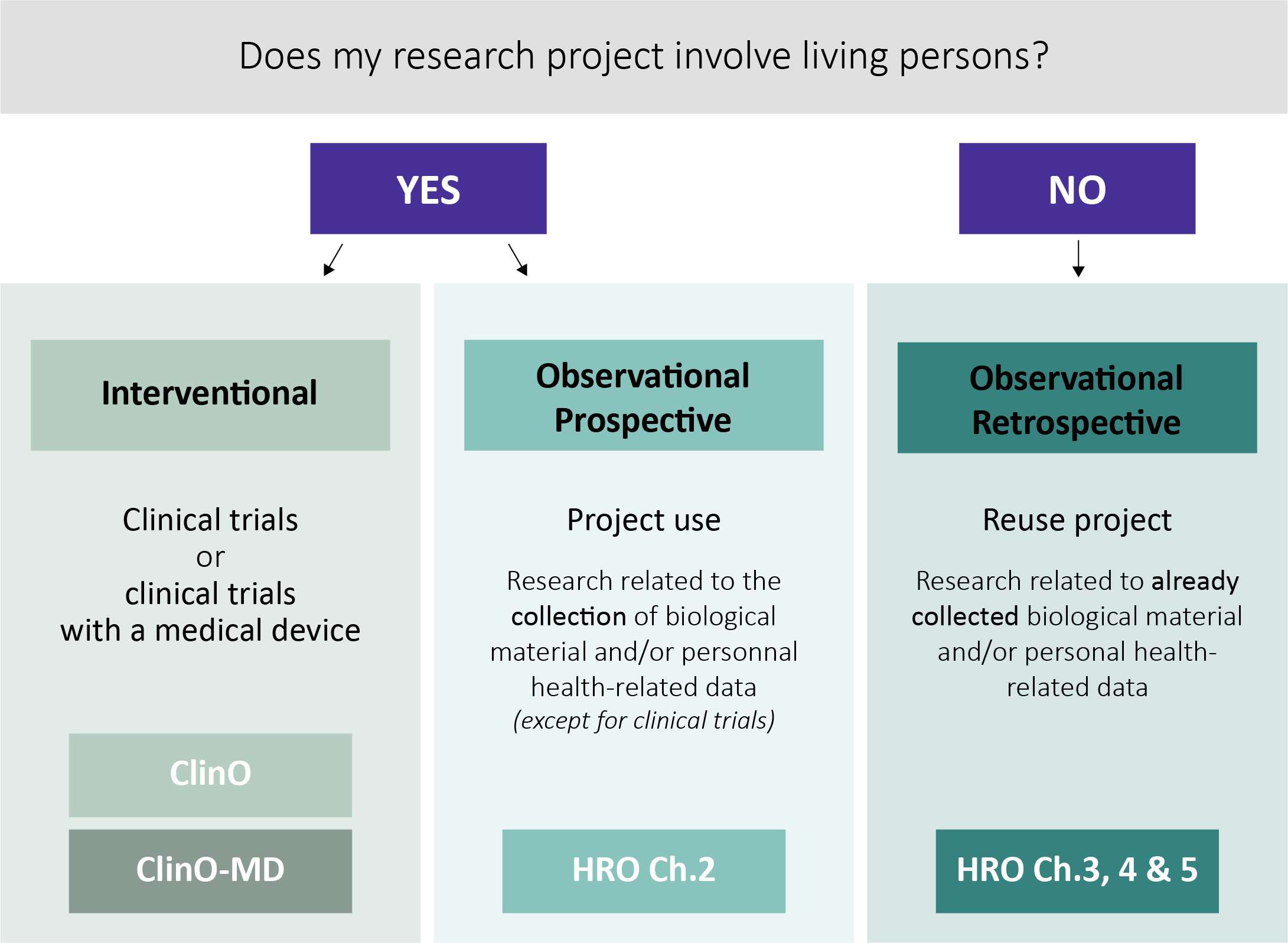
The ordinance on clinical trials in human research (ClinO) regulates human research projects classified as clinical trials.
The ordinance on clinical trials with medical devices (ClinO-MD) regulates in particular clinical trials with medical devices, with the exception of in vitro diagnostic devices, devices in accordance with Art. 2a, para. 2 TPA and combinations of devices and medicinal products (in accordance with Art. 2, para. 1, let. f and g ClinO-MD). These exceptions are governed by the ClinO.
The ordinance on research involving human beings (HRO) regulates all human research projects with the exception of clinical trials.
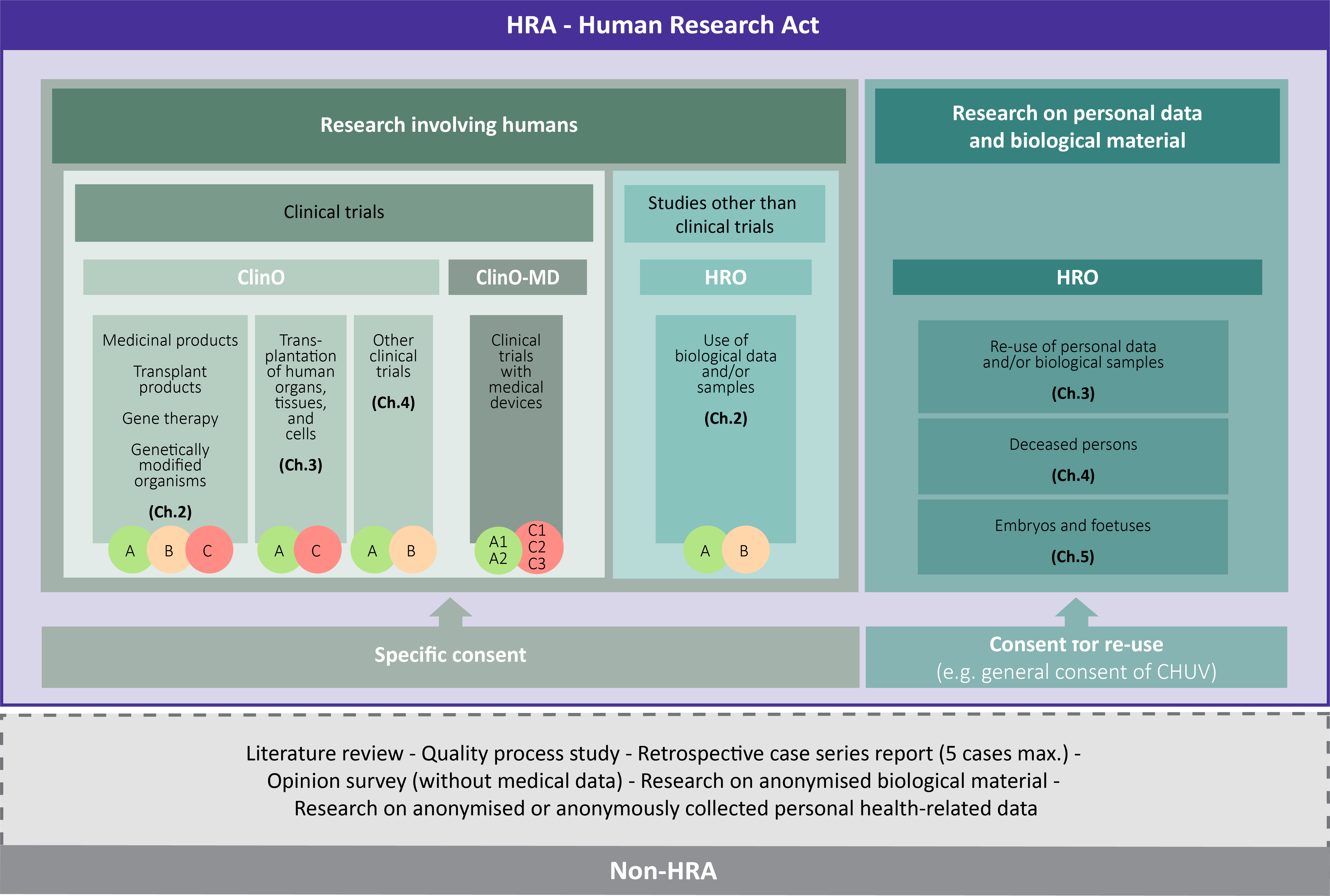
If my research project does not fall within the scope of the HRA, a submission to the ethics committee is not required, so what procedure should I follow?
To obtain confirmation that your project does not fall within the scope of the HRA, you should contact one of the following entities:
• CHUV Legal Department (afj9@chuv.ch) or the legal department of your institution
• Competent ethics committee (CER-VD)
For any investigation at the CHUV involving healthcare, please contact the Commission d’évaluation des demandes d’enquêtes (CEDE)

