
|
|
|
Facility News
|
Hello all ! We are finally in pumpkin spice latte month ! There's nothing like a 1000 Kcal caffeinated drink to boost you through your long experimental days. Please indulge in the sweetest drink of the year and get your monthly fix of flow news !
|
|

|
This month, we talk about the proper set up of Voltages or "Voltration". It is crucial for your data as it will ensure that no valuable population is left behind in the background noise of our fluorescence measurements.
|
In the Flow Post-it ! I would like to refresh your knowledge on sample preparation and the importance of single cell suspensions not only on the quality of your data, but also the smooth running of your samples.
|
Finally, we are offering a FlowJo Seminar & Training on October 19th at both the Biopole and the Agora sites. Please contact Nicolas Gesterman (Biopole) or Mara Kornete (Agora) for more details about attending on site. The training will also be accessible via Zoom so there is really no reason to miss this opportunity to up your game in data analysis !
|
|
FACS Tips
Voltration
Setting Proper (PMT)-Voltages
Changing and setting voltages is a crucial part at the start of any experiment, because once we set our voltages they cannot be corrected afterwards. This means you might be left with awkward looking plots if care isn’t taken beforehand. By performing a proper voltration at the beginning of our experiments, we can optimize our voltage settings and make the best plots. The voltage value on a cytometer represents the degree of amplification of the signal coming from the detector. The goal while setting it is to get the greatest amount of separation between our positive and negative, have our negative sit above the noise of the system, and avoid running our sample off scale. While we tend to approach this by just going by eye, sometimes a more thoughtful approach is necessary.
|
A Brief Overview on Signal and Noise
Each measurement in flow cytometry is composed of signal and noise. Noise is generally a result of the hardware components, both optical and electronic, but can also originate in the sample from autofluorescence or free fluorophores. Electronic noise is produced by, amongst other things, photon to photoelectron conversion, current to voltage conversion, and/or amplification. Every channel on a cytometer will show some degree of electronic noise, some channels more than others, and it can be inconsistent from one day to the next. Autofluorescence is due to the structural elements in the cells and can change based on the stress or activation state of a cell. While they both diminish the ability to resolve signals, electronic noise and autofluorescence are different influences. Autofluorescence for example is particularly visible in the BV510 and FITC channels, but very little is seen in the long red channels such as APC or APC-Cy7.
|
Voltration
|
A voltration is the procedure of adjusting the voltage of the detectors such that an optimal signal intensity is measured above the noise. There are a number of ways to perform a voltration, some quite simple, others requiring the use of special calibration beads. The good news is that most times little to no changes need to be made to our voltages. The tri-weekly CS&T calibration on the machines sets the voltages such that a dim population sits above the noise of the machine and a bright signal is on scale; specifically it adjusts the signal of the negative beads until the robust SD are 10x the robust SD of the electronic noise. Be aware though, that sometimes this CS&T voltage setting can be too high and run bright signals off scale.
|
For her thesis1 Evaluation of Voltration Approaches for Optimal Data Acquisition in Flow Cytometry, Meredith Weglarz compared 8 techniques for optimizing voltration (listed below), and determined the two most useful, which are described in more detail.
|
-
|
Negatives are centered within 1st decade
|
-
|
Negatives are centered at 10^2
|
-
|
Using instrument generated voltages determined by CS&T
|
-
|
2.5 x rSDen (Instrument Generated)*
|
-
-
|
Optimize with stained cells*
|
-
|
Optimize with Stained capture beads
|
-
|
Stain index of Peak Two and Six Beads
|
2.5 x rSDen (Instrument Generated)
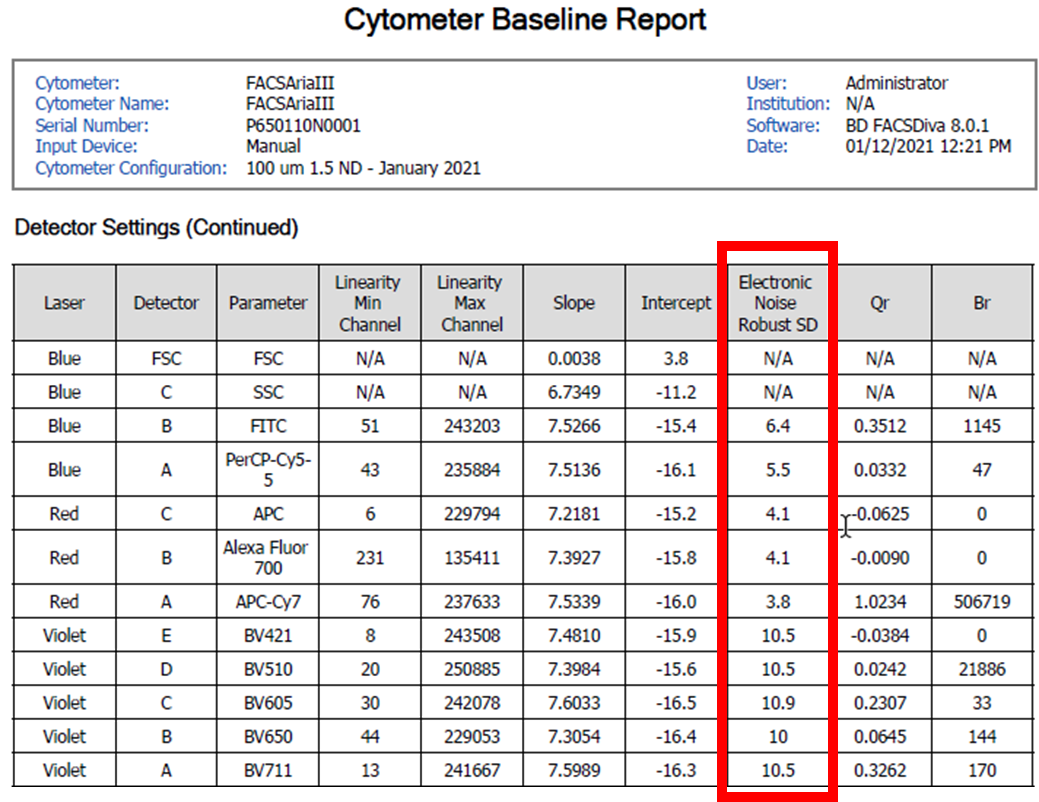
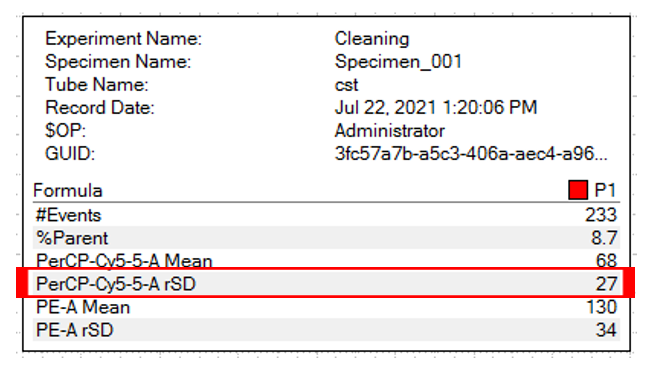
Considered the gold standard for instrument setup, although time consuming it does generate good results. The user must adjust voltages such that the rSD (robust standard deviation) of the negative population is at least 2.5 x the instrument generated rSDEN (robust standard deviation of the electronic noise). The rSDEN can be found in the Baseline Report as part of the Performance Tracking link under the cytometer tab. The rSD for the negative can be determined by creating a statistics view in your experiment worksheet.
|
|
|
|
|
|
The robust SD electronic noise determined from the Baseline report for each detector (Left), and the negative population robust SD determined from the statistics window in the worksheet (Above).
|
Optimize with stained cells
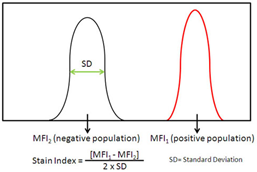
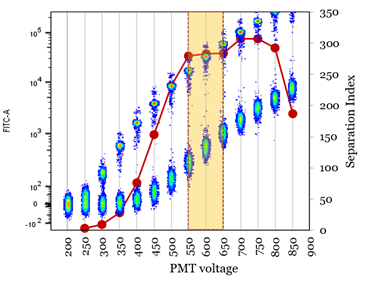
This requires preparation of single stained cells for every channel, and optimizes based on the stain/separation index. Performed much like a titration, however, rather than changing the antibody concentration you change the voltage until the highest stain index (red line) is determined.
|
|
|
Image from https://www.biolegend.com/en-us/blog/the-stain-index-what-is-it-and-what-does-it-tell-you
|
|
Image from https://www.uth.edu/imm/service-centers/flow-cytometry/pmt-voltage-optimization
|
Conclusions
Presenting a plot with cells off axis, or hidden behind the noise is a bad way to display the success of your experiments. By setting up accurate voltages before an experiment we give ourselves the best chance to produce good looking plots and most accurately measure our populations. Feel free to ask the FCF staff if you need any help preparing a voltration experiment.
|
1 Weglarz, M.. “Evaluation of Voltration Approaches for Optimal Data Acquisition in Flow Cytometry.” (2018).
|
|
|
|