|
|

|
|
|
|
|
Facility News
I know it's October but just how we say in France "On n'a jamais été aussi près de Noël !" (We have never been closer to Christmas!) and this month is going to deliver in that department !
|
For some unknown reasons (potentially, my constant obsession with it), the Aurora has been really busy in the past few months and you have been asking for a second machine to help with the work load. Wait no more ! A second Aurora will soon be present in our facility in Epalinges. I hope this news will enlighten your month !
|
|
In addtion to the Aurora, In this month FACS Tips, we are previewing a new sorter we will soon have in our facility in Epalinges. The FACS Discover S8 is adding new flavors to your sorting with imaging and spectral cytometry capabilities ! Please check it out 😊
|
|
Emina Dzafo won the mug this month, Congratulations !
|
|

|
Each month, we will give away one of those special and unique mug designed by the FCF team. Answer correctly to our 3 questions and you can have a chance to win !
Please take few minutes to answer the quiz HERE.
|
|
|
|
|
|
FACS Tips
|
FACS Discover S8
|
Francisco and Kevin had the privilege of traveling to BD headquarters in Heidelberg back in September to get a demo of one of the newest innovations in flow cytometry, the FACS Discover S8. A first of its kind instrument, combining imaging, high dimensional spectral analysis, and sorting, all in one. This machine presents an exciting research opportunity, and we were very excited to get to test it in person.
|
|
Because it’s a spectral machine, like our Cytek Aurora, it’s no longer limited in fluorophores to the number of detectors like all our current sorters, but rather by the number of unique signals included in your panel. It has 5 lasers and a massive 78 detectors (APDs) as part of its configuration, meaning panels of beyond 40 colours are conceivable. This allows you to sort deep into cell lineages in a way we couldn’t have achieved before. For users interested in complex cluster analysis, it will be possible to set up your panel on the machine, analyze a sample and use the HyperFinder cluster tool in FlowJo, then import that analysis back into the S8 software and sort based on clustering as opposed to traditional gating. This opens up the possibility of sorting novel cell types that couldn’t have been collected before with conventional gating strategies.
|
|
|
|
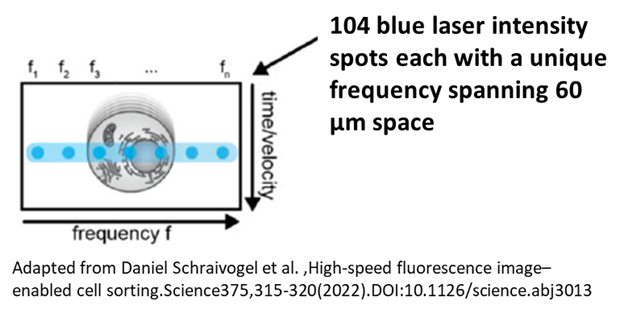
The imaging system on this machine is very interesting. There is no camera like on the ImageStream, but rather the blue laser is split, one part to perform the typical blue laser excitation to be sent to the APDs for spectral analysis, and the other part broken up into 104 spot excitation points stretching across the 60 µm stream, each with a unique frequency. You can imagine, as the cell passes across this array of spots, it scans the cell, exciting with the blue laser at all these different locations. So, when any emission is generated from this it’s captured on a separate set of PMT detectors and then deconvoluted for its frequency, relating to its particular spot, as well as the time as the cell is moving through the stream to construct an image. This means though, that we can only generate images off the blue laser, and only in three fluorescent channels corresponding roughly to FITC, PE, and PE-Cy7. On top of this though, you also get a FSC, SSC, and Light Loss (Brightfield) images. From these captured imaging parameters, including the expected intensity of the fluorophore, many other features are calculated that you can see listed in the figure below. Suddenly we have a massive amount of spatial information that we can use to our advantage in experiments creating a variety of new possibilities. You can even sort based on image parameters which is very exciting.
|
|

|
While not nearly as ground breaking, this sorter will add the ability to sort up to 6 populations at once, and has some nice features that us in the facility will particularly appreciate.
|
|
Thanks to the help of our user base we had a couple of test samples to try. In one you can see GFP tagged inflammasome samples. When the inflammasome forms, the GFP gathers from a diffuse signal into an intense spot. With this machine we can now see and sort on these cells in a way we couldn't before.
|
|
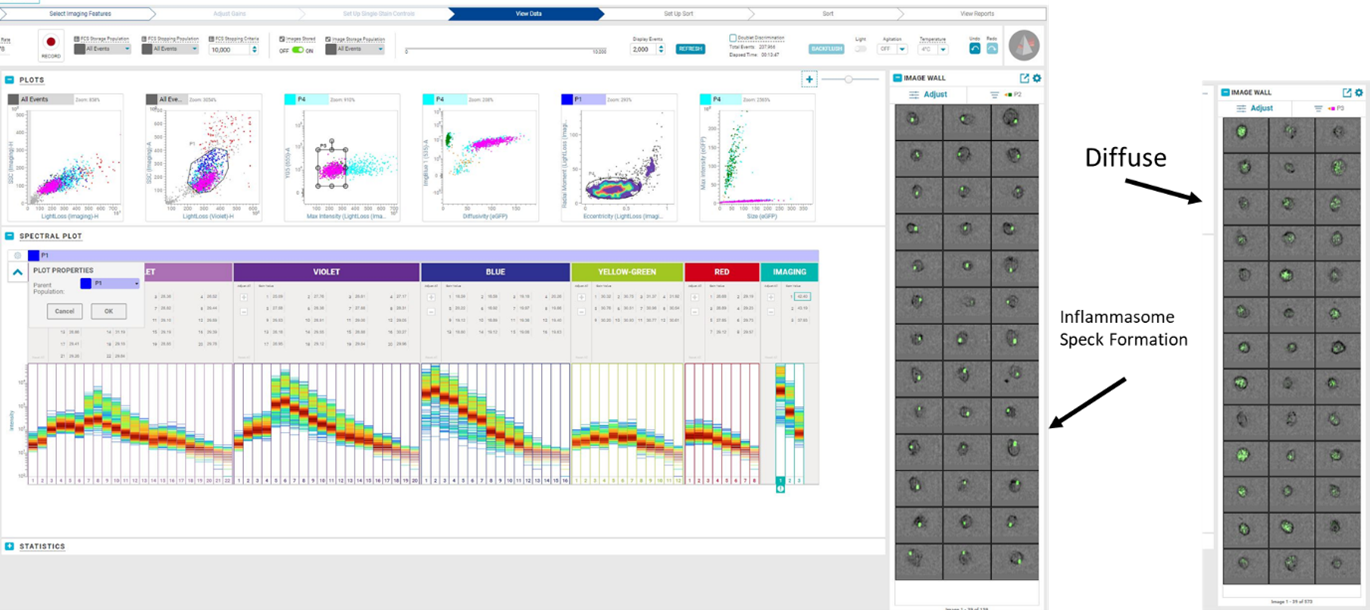
|
Click to enlarge
|
|
Where things get more complicated, at least at the moment, is when we get to the analysis. You’ll definitely need to update to the latest version of FlowJo, also you’ll need to add the CellView Lens plugin for FlowJo, as well as an additional application called Image Extractor to extract the images you’ve taken and load them into FlowJo. Once in FlowJo, there is also now the added complication of an addition of several hundreds of parameters in your drop-down menu, making things quite confusing to work with. I’m sure this will be corrected over time, but it’s quite intimidating at first glance. These added parameters reflect not just Height, Area, and Width parameters for each of the 78 detectors, but also parameters for each of the imaging channels, along with other imaging parameters. Taken together this makes it very difficult to do your own analysis since we’re no longer dealing with just marker intensity. I expect BD to rollout a comprehensive guide for imaging analysis and this will take some time, but to do it on your own is daunting.
|
|
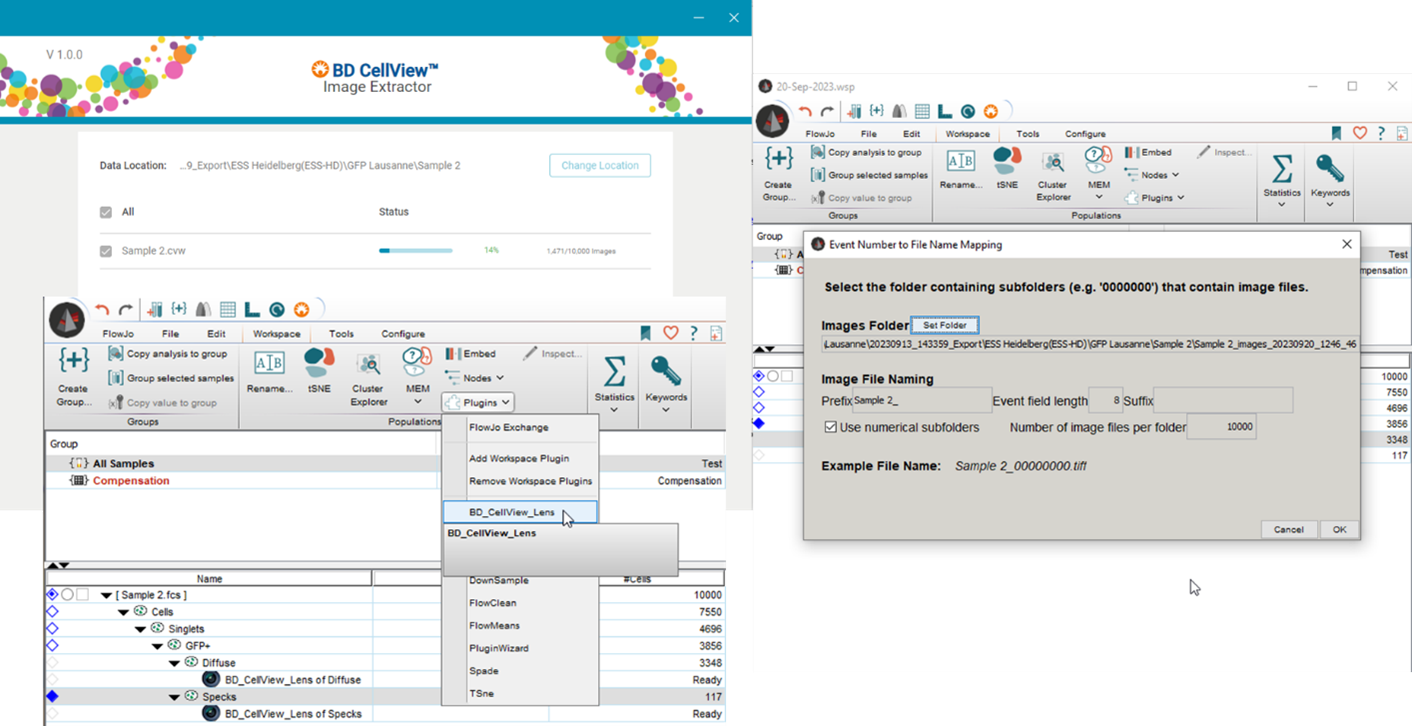
|
Click to enlarge
|
The upfront sorting end might be more challenging for us too, and it will take some adjustment to use. There is a balance of getting both all the spectral unmixing right, and correctly setting the imaging parameters for capture. Combined, these two steps will certainly take more time to prepare than your typical 5 colour sorts where we can often get by with no or old compensation, for which this may no longer be the case. Also, the machine no longer runs on the familiar FACS DIVA software but rather FACS Chorus. At the moment of the Demo there are still some features you would expect that are missing. For instance, it was not possible to append a recording on a tube, and some of our more common sorting features are missing. BD has said these are all expected to be added in the near future though, as they will provide software updates to meet user demands over the coming 6 months.
|
In conclusion, we’re super excited to see what this machine can do, and all the possible experiments it will open up. It certainly won’t replace some of our simpler machines capable of more efficiently hammering out some of our more straightforward experiments, however it will open a new field of applications of cell sorting where it will be possible:
|
- To improve the cell sorting quality for 10x genomics experiments with a better discrimination between artifacts and cells of interest
- To sort cells based on fluorescence localisation/co-localisation
- To sort doublets of interacting cells
As always, feel free to reach out to the FCF staff if you have any questions.
|
|
|
|
|