|
|

|
|
|
|
|
Facility News
|
Just as the kids got back to school, you gotta be back to work and a new wave of master students an/or PhD students are waiting for your time and expertise ! Being a mentor never stops, but we can at least cover the flow cytometry part ! Good luck and enjoy one of the last week of summer ☀️ before the fall 🍂 !
|
|
In this month FACS Tips, we are revisiting tandem dyes and how their unique chemistry implies even more consideration when you are using a spectral machine instead of a conventional flow cytometer.
|
|
Sarah Boillat won the mug this month, Congratulations !
|
|

|
Each month, we will give away one of those special and unique mug designed by the FCF team. Answer correctly to our 3 questions and you can have a chance to win !
Please take few minutes to answer the quiz HERE.
|
|
|
|
|
|
FACS Tips
|
Tandems vs Time and what that means for Spectral Unmixing
|
|
Back in January of 2022 we published a newsletter on Tandem Dyes. Though it's certainly worth revisiting for a more detailed look at tandem dyes, the key message was that while tandems have become an essential part of any large panel experiment, they also introduce unpredictability. That is because they are prone to degradation, and also that their construction both between separate vendors, or even between batches of the same vendor, can be slightly different, changing their fluorescent properties. Normally this isn’t a problem though, as long as we rerun our singles stains often, keeping our controls up to date with the vendor and batch of our fully stained samples. Unfortunately, we often fall into the trap of reusing old single stains a little too long creating compensation errors. On conventional cytometers this doesn't appear to have too great of an effect, its more simplistic compensation calculation isn’t necessarily as affected by minor deviations in the fluorescence of tandem dyes as it is only really concerned with the one primary channel/detector per fluorophore. This is not the case though for the much more complicated spectral unmixing algorithm.
|
|

|
Spectral cytometry is about taking the entire fluorescence spectrum of a fluorophore and using it as its own unique signature to separate it, or unmix it, from all the other fluorophores in a sample. This way our panels are no longer limited by the number of detectors on a machine, but rather the number of unique fluorescence signatures we can include. The focus is no longer on just the maximum excitation peak, but also the secondary and tertiary peaks of a fluorophore that make up its unique spectral pattern. The unmixing relies on these various peaks and valleys across the entire length of the detector range to be consistent between the single stain control and fully stained sample, otherwise it’s much more complicated unmixing algorithm will produce errors. And this is where tandems can become a problem.
|
|
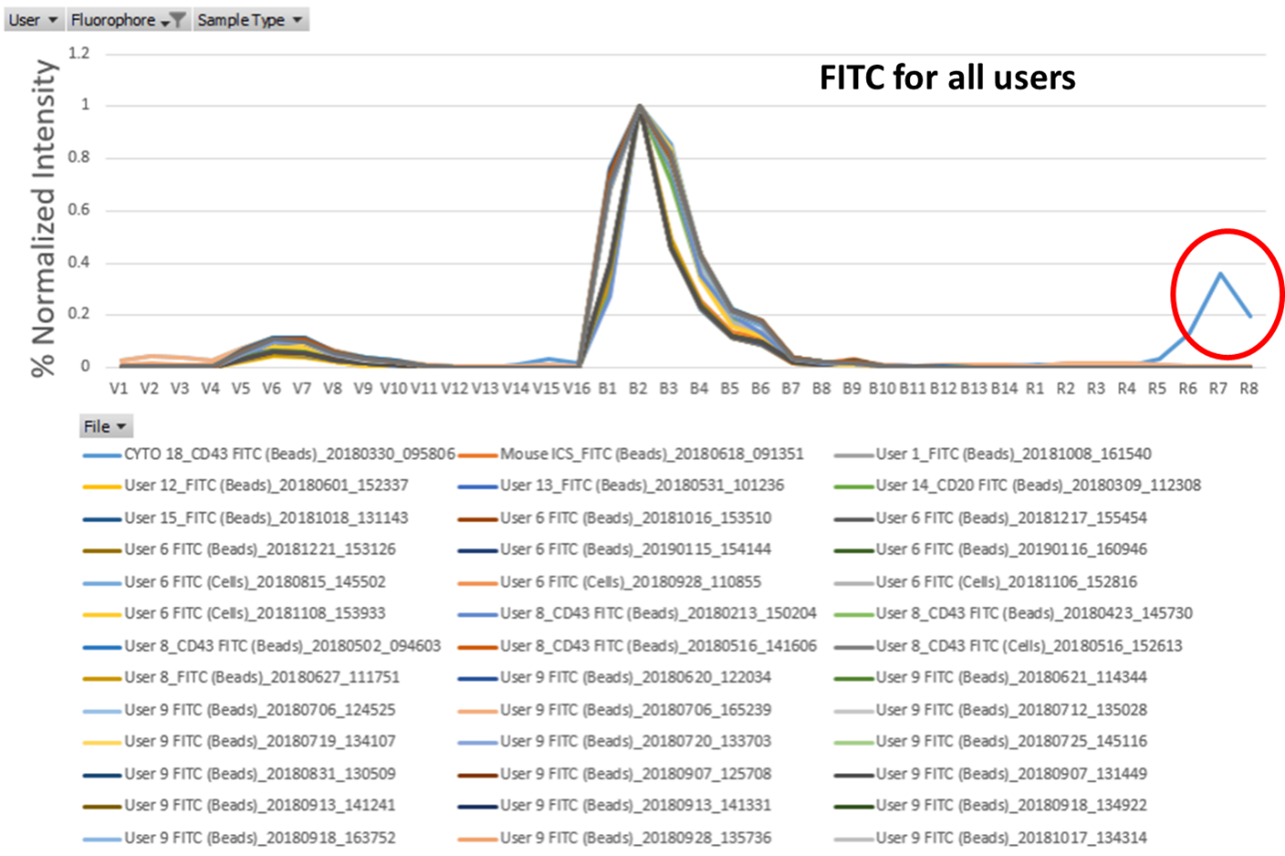
So, we know spectral unmixing is a more complicated algorithm that is more susceptible to small fluctuations in fluorophores upsetting our data, and that tandem dyes have a habit of unpredictability that can create these fluctuations in the first place, but what does this practically look like? While we have a reference spectrum sheet to check against at the machine, it doesn't perfectly reflect both your previous samples and our machine. A great demonstration of this can be found in a tool created by Alex Wendling at the University of Virginia. He has put together a library of single stain spectrums recorded on their Cytek Northern Lights 3 laser spectral machine with the goal of tracking reference spectrums to see if it was possible to create a universal reference control. The spectrum of each single stain is plotted across all the machine's detectors and normalized to the max peak emission. You can check it out HERE.
|
|
|
|
Using this tool we can see how non-tandem reagents have quite good stability over time, such as FITC. Across all the users, and with both beads and cells there appears to be a pretty clear consistent spectrum. One user does appear to have a contamination in their spectrum though in the red detectors, which is another advantage of this tool as it can show where some reference controls can go wrong.
|
|
|
|
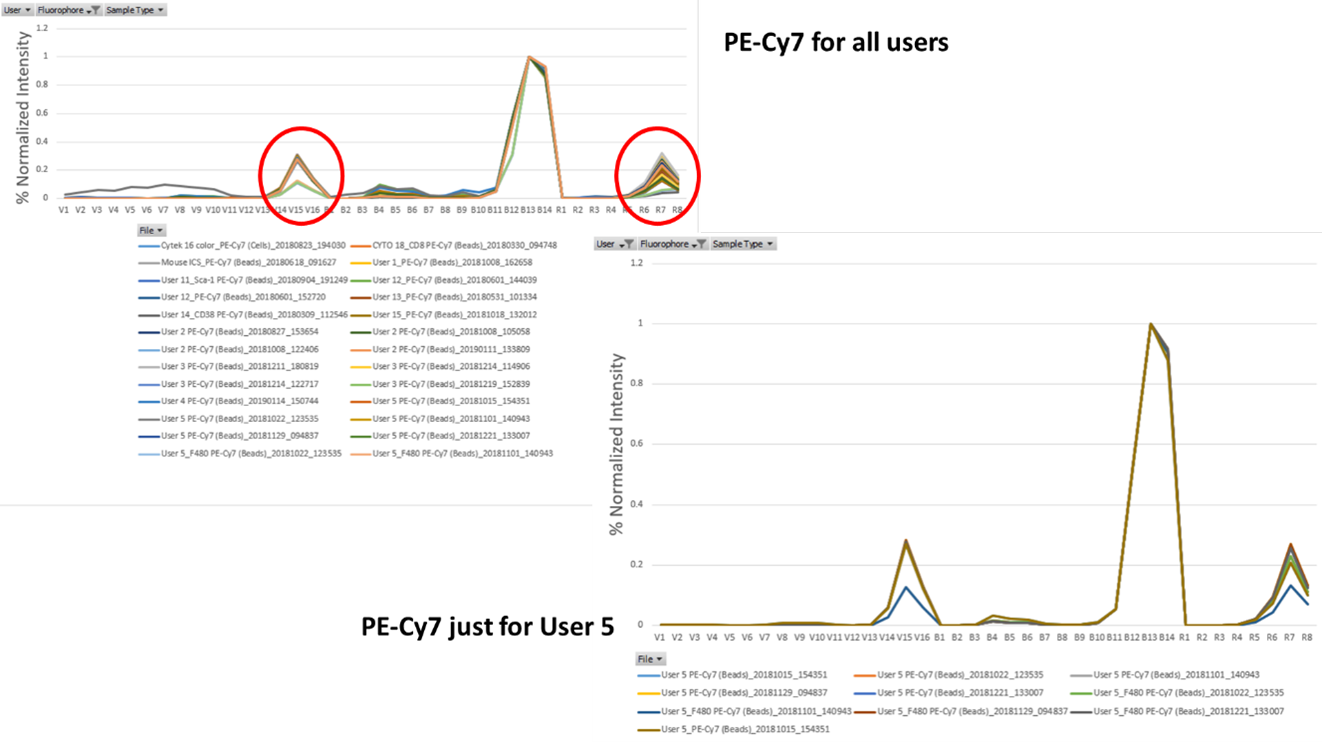
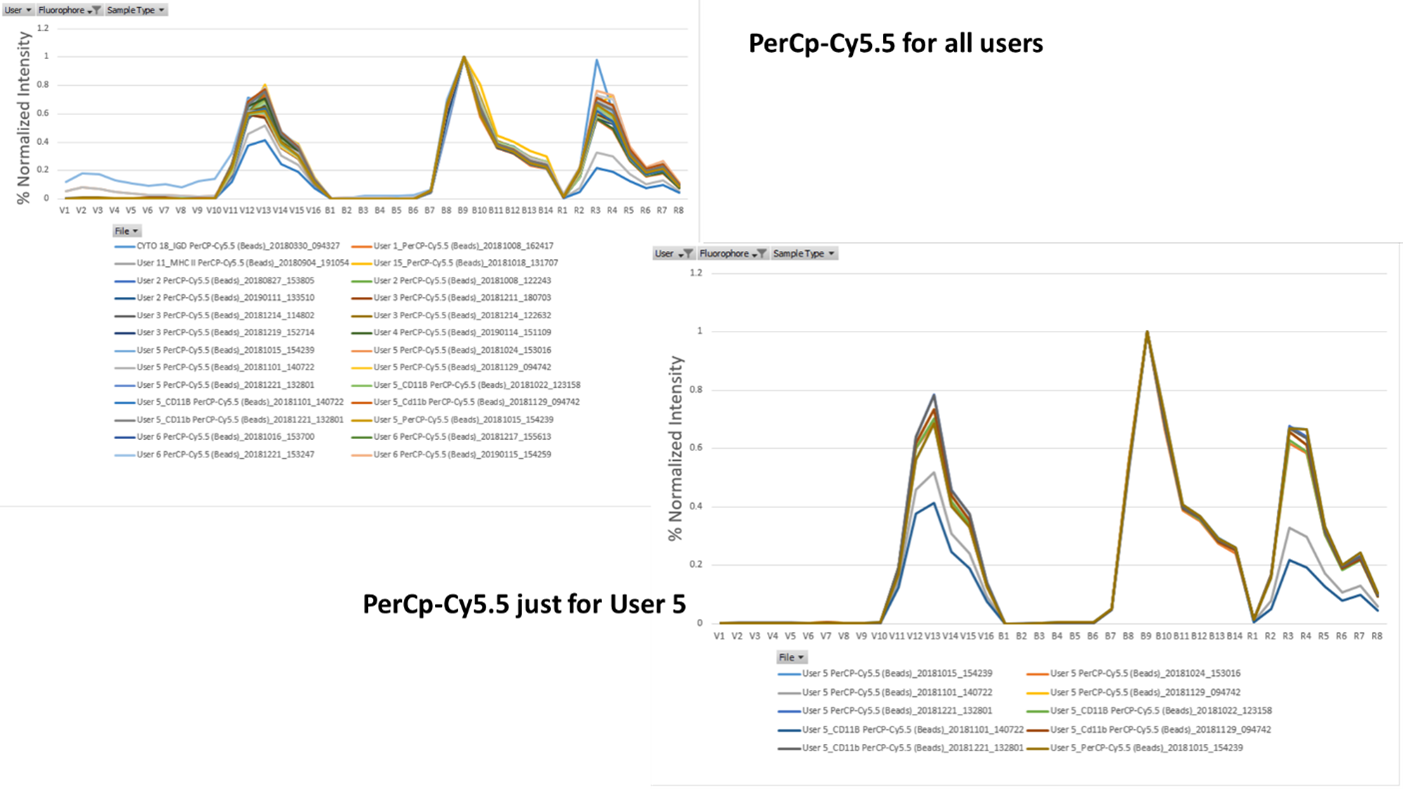
Comparatively, if we look at the chart for PE-Cy7 we can see much more variability. While the primary peak at detectors B12 and B13 is quite consistent, the secondary and tertiary peaks are more variable. When we selected just User 5, it really illustrates how even just one user can have a range of different spectrums across their various vendors and batches of single stains for PE-Cy7. Same is also true of PerCp-Cy5.5. While its primary peak is quite consistent, there is more variability around the secondary and tertiary peaks. These small changes can introduce errors into your unmixing algorithm on spectral machines. If an old single stain is applied for the unmixing that has quite a degree of difference from the current batch, suddenly your plots can have a distorted effect that you wouldn’t expect.
|
|
|
|
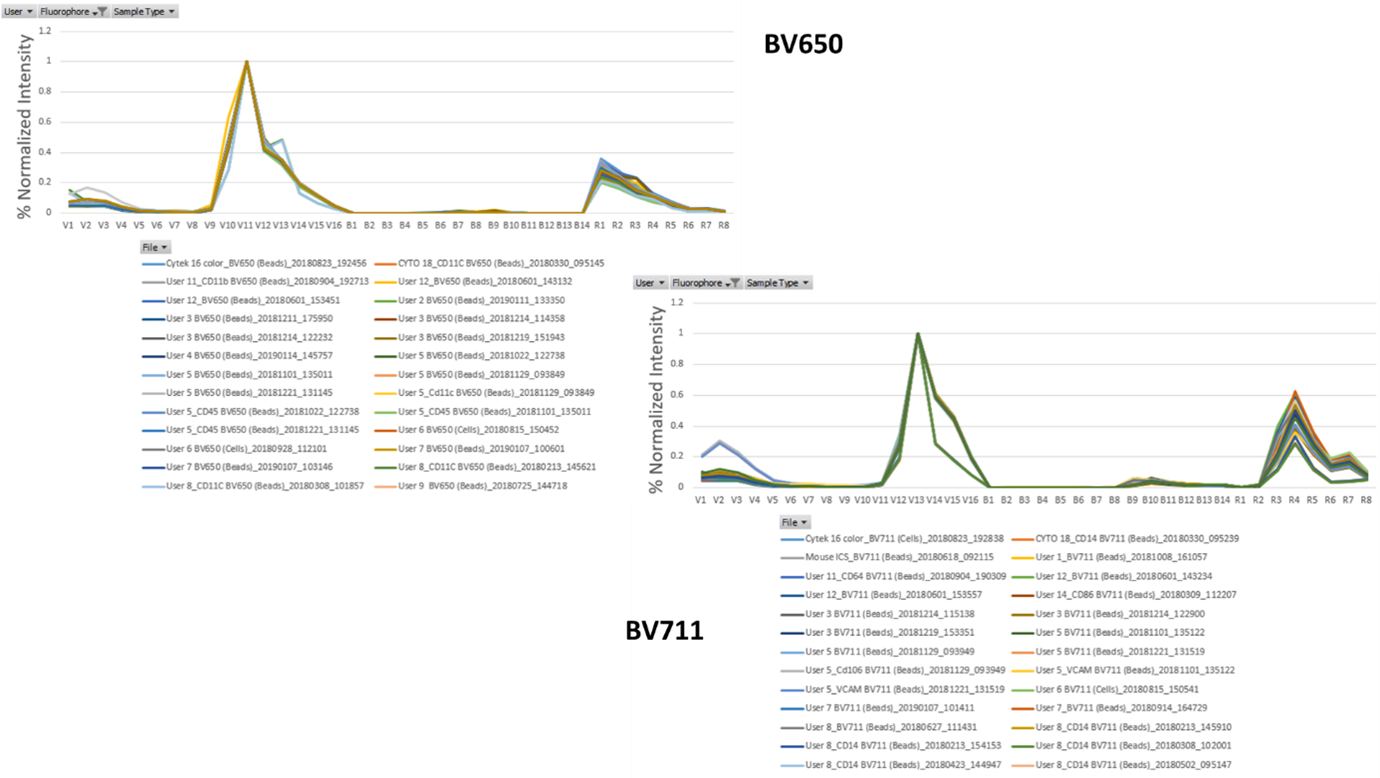
This is also true of the more modern brilliant violet polymer tandem dyes. Although their synthetic construction allows for a fixed donor to acceptor ratio compared to protein-based tandems, it still does exist and you can see it in the examples for BV650, and BV711.
|
|
|
|
This isn’t meant to say these are bad reagents, or that you should avoid using them, only that they have an observable degree of variation across time and batches, and we need to make sure our single stain controls stay up to date to match this. If you think your plots are distorted, but are sure of what possible problems could be causing it, consider how old the single stains you applied for your unmixing/compensation are. It could be the source of your problems. As always, feel free to reach out to the FCF staff if you have any questions.
|
|
|
|
|