|
|

|
|
|
|
|
Facility News
|
It was only few days ago that we celebrated Swiss National Day ! But it is never too late to thank this beautiful country for welcoming so many scientists from all over the world to achieve great Science and great Flow ! So thanks Switzerland ✨🔥 !
|
|
In this month FACS Tips, we are discussing how to make sure you can see your cells correctly with proper FSC and SSC settings. No bigger problems than not seeing cells in your first gate, so please read carefully and don't be afraid of losing them next time around !
|
|
Pierpaolo Ginefra won the mug this month, Congratulations !
|
|

|
Each month, we will give away one of those special and unique mug designed by the FCF team. Answer correctly to our 3 questions and you can have a chance to win !
Please take few minutes to answer the quiz HERE.
|
|
|
|
|
|
FACS Tips
|
Troubleshooting with FSC, SSC, and Threshold
|
|
It’s a troubling feeling to put our cells on the cytometer and not see what we expect. A scatter that's unfamiliar to us, and maybe a small amount of panic of what to do next. If you have what looks like your cells in the P1 gate, but nothing appears to be stained it can seem like something deeply wrong has happened with your samples. It’s important in moments like this to resist the urge to just push through and hope that things will look better once we move the experiment to FlowJo. Oftentimes if we didn’t get the scaling right, no amount of adjustment in a post analysis software will fix it, and then you will be left to explain why your cells of interest are pushed off the axis, or cramped down in the corner. It’s possible though, that with just a simple fix of the FSC, SSC, and threshold you can get your cells back on plot, and experiment back on track.
|
|
|
A problem we run into every so often is a user can’t seem to understand why their fully stained sample appears to have no signal in most of the channels, but the user is certain they are stained with everything. A quick look at the FSC and SSC voltage can reveal the problem. Voltage settings, or gain for the cytoflex, is simply the amount of amplification of a signal detected by the PMT or APD. Adjusting these values has no influence on your cells themselves, only an altering of the way their fluorescence is perceived. With FSC and SSC voltage turned up too high, dead/debris events can be pushed into the location where we would normally anticipate our cells on the FSCxSSC plot. This can give the appearance of real problems with your sample, when in reality your cells of interest have just been pushed off the axis. The simple solution to this is just to decrease the FSC and SSC voltage values. Really anytime you have the impression your cells of interest are lost on a messy plot, just decrease the FSC and SSC values by quite a bit, until you have all your events within the axis of the plot, then by slowly increasing the values you can track down your cells of interest. This can also be aided by backgating from a downstream population onto your FSCxSSC plot.
|
|
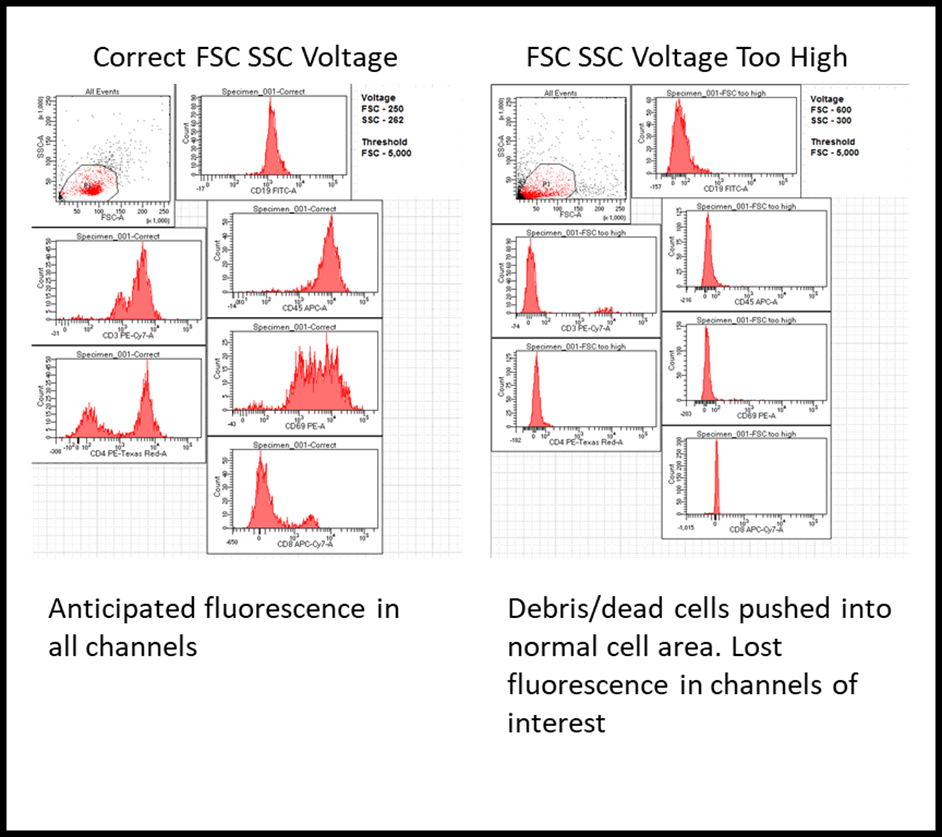
|
|
It also helps to go into a flow experiment with a background idea of what to expect as far as the scatter goes, for instance, neutrophils, with their large size and high granularity will be much higher on the SSC scale than smaller less intracellular complex T cells. Cell lines tend to be larger than primary cells, and may require a decrease in FSC if the two were run side by side. And if your sample is particularly precious with very few cells, it can be especially difficult to determine these FSC and SSC values, because we don’t get enough cells quickly enough to make an adjustment. In this situation it’s helpful to bring a more concentrated control of a similar cell type to perform the set up ahead of time. It’s important to not assume that the FSC and SSC will remain identical between experiments, and should be checked each time.
|
|
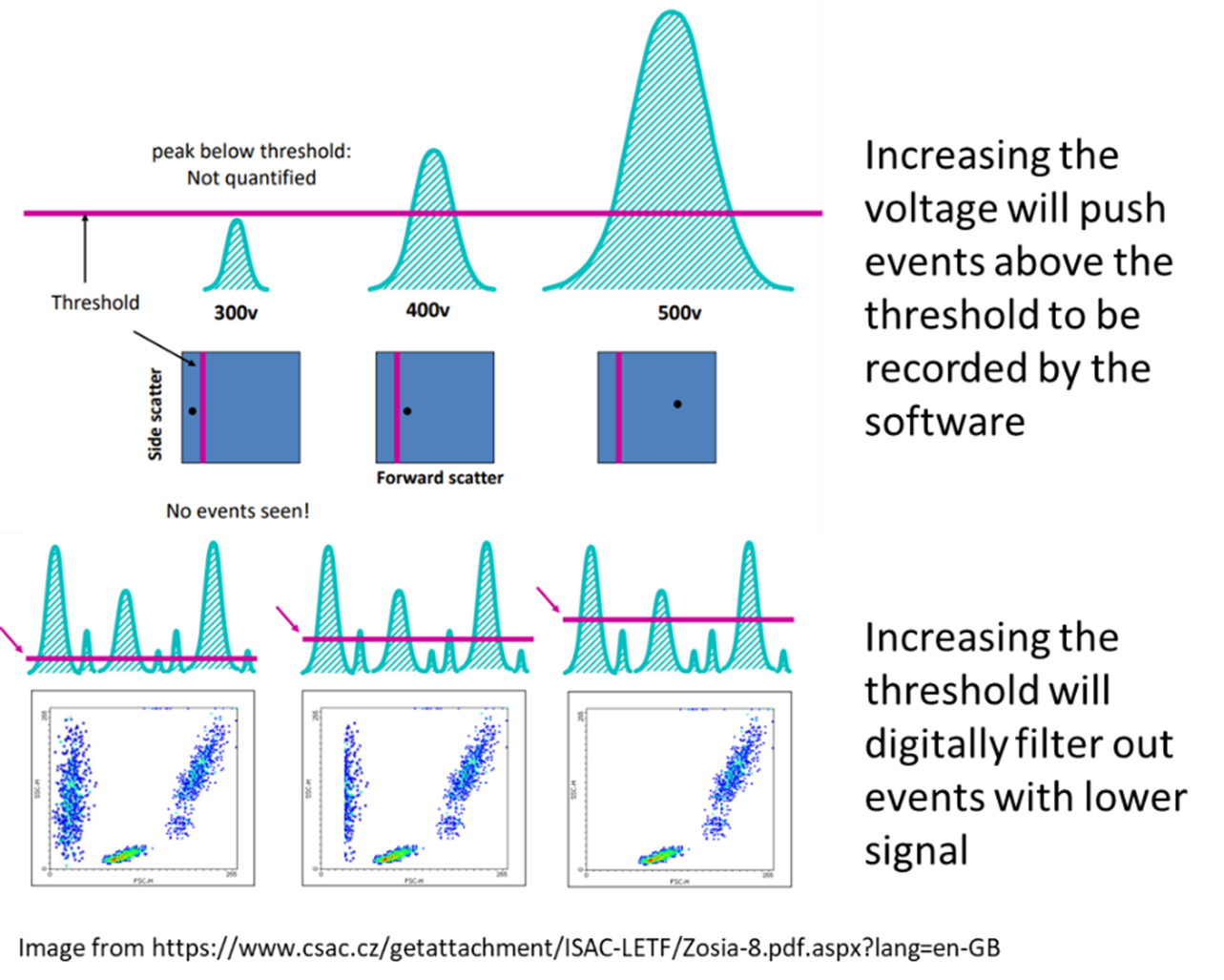
|
|
Another important feature is the threshold value. Threshold is a parameter that can be adjusted to digitally remove any events below a set intensity. On the BD instruments by default it is 5000 off the FSC, but its triggering channel can be changed to another fluorescence signal of your choice, or even several used in combination. This allows us to avoid recording unwanted data as anything below this set threshold will not be collected during analysis. For example, if you have done a red blood cell lysis on a sample and it has a lot of debris, it can be of value to exclude that debris by increasing the FSC threshold. However, if by accidentally increasing this too high, you can also lose your cells of interest. Also, just because the debris is no longer visible on the software doesn’t mean its not there. For instance, in sorting, we have to keep the threshold low to ensure sort purity remains high, otherwise artifacts blinded to the machine would be sorted along with our cells of interest without us knowing.
|
|
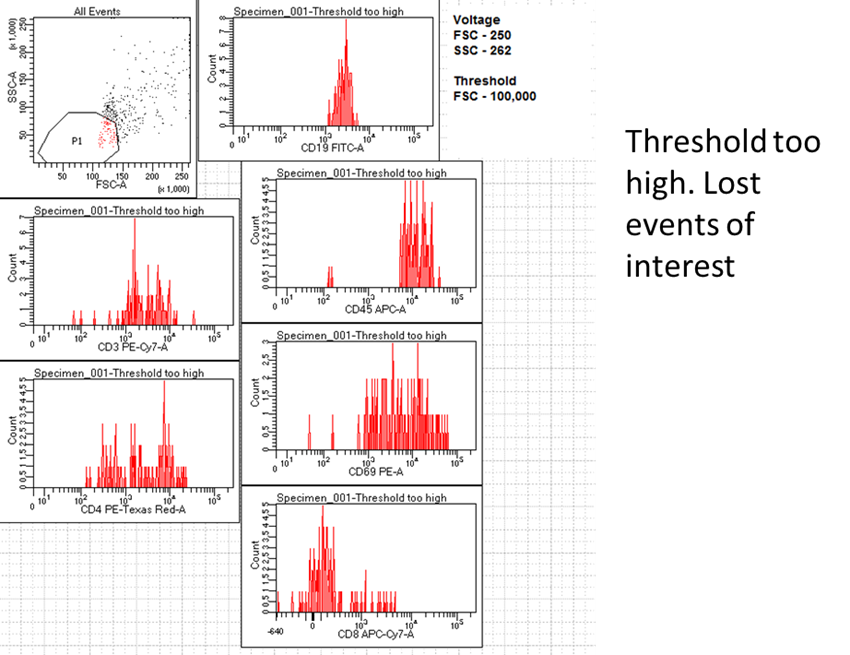
|
|
Sometimes it’s the simplest things that we overlook that can cause us problems in our flow experiments, and FSC, SSC, and threshold are good examples of that. Accidentally getting these values wrong and we can give ourselves a lot of problems when it comes time to eventually analyze. If you see something that looks wrong, take your time and test out different values to try and put your cells of interest back on scale, start with lower values and increase until things look correct. If you’re ever unsure don’t hesitate to ask a member of the FCF staff.
|
|
|
|
|