|
|

|
|
|
|
|
Facility News
|
We are in the last leg of the "Let's-spend-as-much-leftover-money-before-the-year-end" race ! This usually equals to busy weeks leading to the end of the year festivities for the FCF Staff and we love it ! Not only do we get to be useful for the community but we are also able to catch up with many users that don't sort as usually as others !
|
|
|
|
As in previous years the Flow Cytometry Facility (Biopole 3 and Agora/CHUV labs) will be officially closed from 6.00pm Friday December 22nd 2023 through to 12.00 noon on Wednesday 3rd January 2024. During this time there will be no FCF support or training, and no possibility to organize repairs as the service engineers from all companies are also on vacation until January 3rd 2024.
Sorting Service
There will be no sorting service at either site from 6pm Friday December 22th 2022 until 12.00 on January 3rd 2023. Please plan your experiments accordingly.
Analytical Flow Cytometers
All analytical flow cytometers will be available for use by experienced users with valid access cards in both the Biopole 3 (AA31 and BA32) and the Agora/CHUV (227 and 233) labs during this time. However, no staff will be available for troubleshooting or help with setting up experiments. Please only use the machines if you really need to. Bookings should be made as usual on the IRIS booking system.
End of year Billing
Please note that the November 2023 billing will include all FCF use up to and including December 15th 2023. The January 2024 billing will include December 16th to December 31st 2023 bookings as well as all of January 2024.
|
|
|
|
In this month FACS Tips, Kevin will explain the relevance of OMIPs and how it can not only save you time and money but also can provide an amazing tool to normalize your data across your team ! Give it a try and never go back !
|
|
Mélanie Charmoy won the mug this month, Congratulations !
|
|

|
Each month, we will give away one of those special and unique mug designed by the FCF team. Answer correctly to our 3 questions and you can have a chance to win !
Please take few minutes to answer the quiz HERE.
|
|
|
Merry Everything to all of you and I hope you can enjoy a nice end of the year break
|
❄️⭐ !
|
|
|
FACS Tips
|
Optimized Multicolor Immunofluorescence Panel (OMIPs)
|
Ever think it’s weird that two groups, could be across the world from each other, could be separated by only a couple doors in the same research building, can target the same group of cells by flow cytometry, but with a completely different panel? How is it that flow cytometry is such a free for all, with little to know universally held panels and markers. There are certainly a variety of reasons, from antibody costs and availability, machine differences, to just particularities with certain researchers, along with many other reasons too long and unnecessary to get into here. But as complexity grows, and panels become more a work of art and precision than a throwaway task to be handled in a rush, some unifying guidance can certainly be of value. With a vision of improving the quality and reliability of scientific publications in the flow cytometry space, but not limited to just it, Cytometry Part A set out to tackle this problem. In 2010 they started a series of publications under the title Optimized Multicolor Immunofluorescence Panel better known as OMIP.
|
OMIPs stated aims are to do the following;
|
- To alleviate the development time for researchers in need of the same or highly similar panels,
- To provide a starting point for the creation of novel OMIPs, and
- To give the developers of the panels credit via citation or the publication.
|
|
To date there are 99 published OMIPs, each with a standard configuration of just a couple pages starting with a purpose and appropriate sample type, short narrative, reagents list, and an example staining figure, which provides the template for any replication gating strategies. On top of this there will be a supplementary online component with plenty more technical details for everything that went into designing, implementing, and executing every stage of the panel development.
|
|
The benefits of OMIPs are immediately clear, instead of the hours and hours of time spent planning and troubleshooting complex panels, instead we can simply just apply the same or similar staining already proven to be successful, without all the hassle. With the detailed reagent lists we also avoid the wasted costs of unneeded or inadequate reagents. As the O in OMIP stands for Optimized, even little things like titration and concentration of antibody will have been performed for these panels. When used appropriately, these OMIPs stand to save researchers a substantial amount of development time.
|
|
|
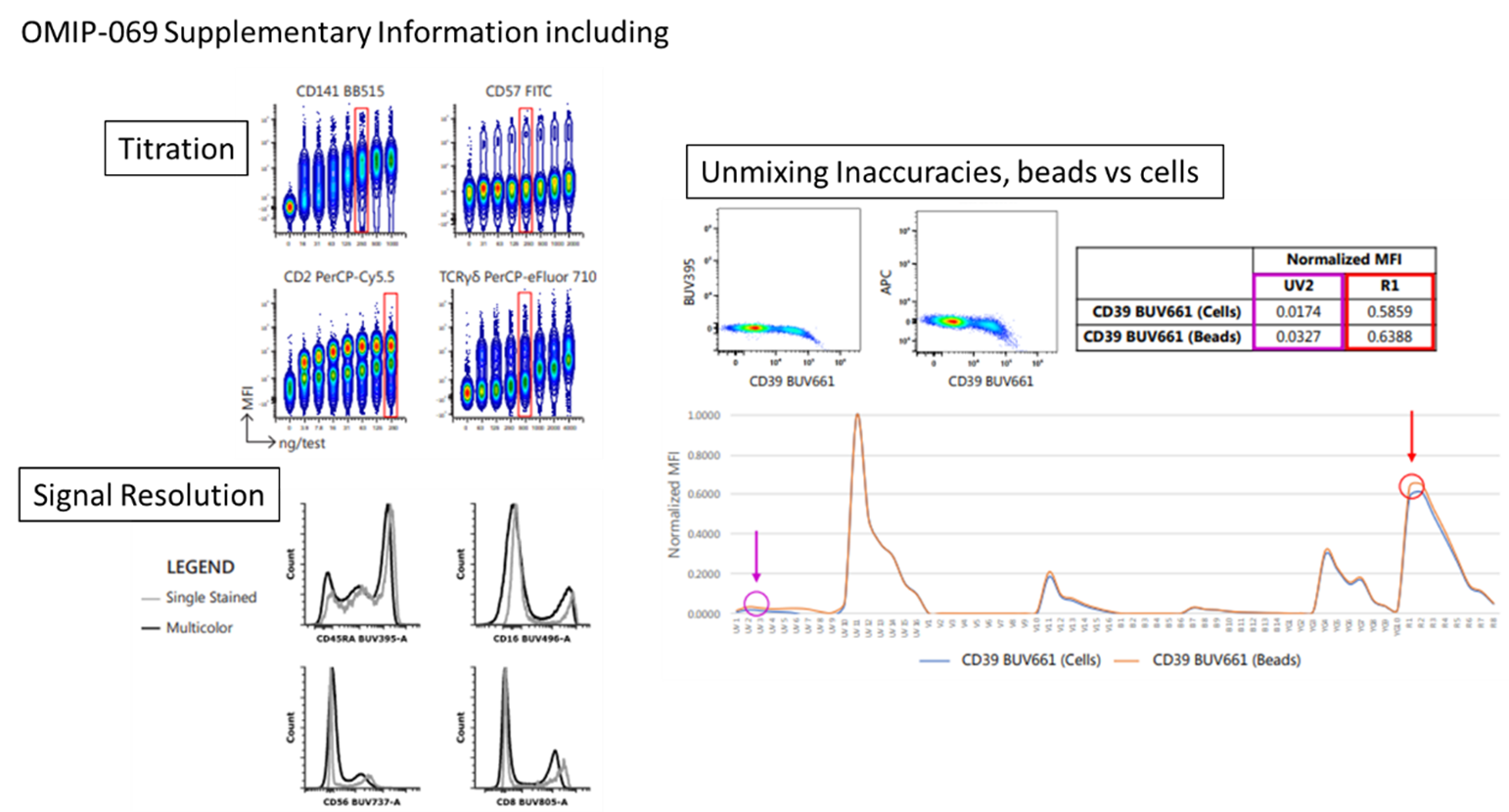
We’ll take one of the best known OMIPs as an example, OMIP-069, which is a 40 colour Deep Immunophenotyping panel designed for Human peripheral blood samples. It provides an in-depth characterization of lymphocytes, monocytes, and dendritic cells simultaneously covering almost all of the cellular composition of the peripheral immune system. This is the first OMIP to go over 28 colours, and to my knowledge is the largest panel OMIP published. Although 40 colours would certainly be an impressive target to recreate, the value of OMIPs is you don’t have to go with all 40, but rather just pick and choose some of the markers that matter to you and use it as a backbone for future panels.
|
|
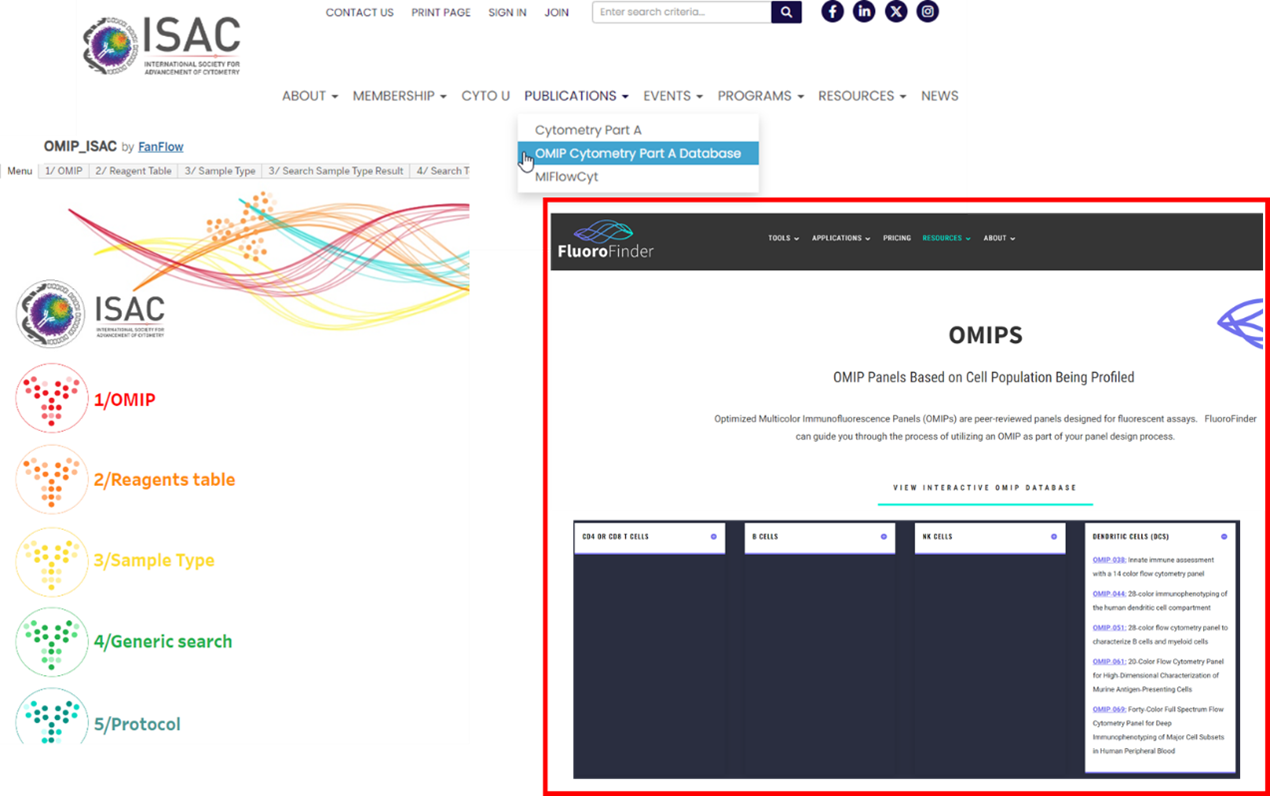
With so many published already, it can be difficult to find an OMIP that may reflect the panel you would like to create. Thankfully, there are an increasing number of resources to help us find the OMIP that best fits our goal experiment. On the ISAC website, under publications, they provide an OMIP database. It offers a variety of ways to find an OMIP of interest, if you know the exact number you can just pull up the OMIP list, but if you’re trying to discover a new one based on a certain reagent, you can search by say CD86, and you’ll see all the OMIPs with that marker. The same options are available for cell population, say if you want to search for ones that include neutrophils, and then it's also possible to arrange them by sample type to compare the different species the OMIPs were prepared in. This makes it easy to get to the OMIP of interest in a streamlined way. Fluorofinder also offers an interactive OMIP database, however it could use with some updating as I think they haven’t added some of the most recently published (https://fluorofinder.com/omips/). It has a drop-down menu for each of the most common cell types and the corresponding OMIPs that feature them. Lastly, EasyPanel, the algorithm driven panel builder we have access to here at UNIL will also source from a database of around 500 papers including OMIPs. So, if your input panel shares 10 antigens or more with any OMIPs in the database it will also suggest it to you as a reference.
|
|
|
|
We shouldn’t have to start from scratch every time we want to analyze a well described group of cells by flow cytometry. OMIPs give us the information to save a lot of unnecessary work up front and get to our answers quicker by following an optimized established protocol. With so many published now it’s quite likely to find what you’re looking for with the help of the various database tools available. As always, feel free to reach out to the staff if you have any questions.
|
|
|
|
|