|
|

|
|
|
|
|
Facility News
The french proverb says "En Avril, ne te découvre pas d'un fil !" which litterally translate as "In April, don’t take off a thread (of clothing)". Well, this time around, french people are wrong (and they so rarely are 😛) as we can finally remove the protection masks, move on and see our neighbors and co-workers face again (or for the first time !). So feel free to sing, dance and give us the crazy flow sample running extravaganza ✨ we've been missing for two years !
|
In this month FACS Tips, we will talk about one of our most interesting machine : the ImageStream. It is very complementary to classical flow cytometry and we will let you know how !
|
|
We got our first ever winner for the Newsletter Quiz ! Congratulations to Morgane Lecointre from the Hanahan Lab ! Please enjoy the limited editionToblerone 🍫 for us !
|
|

|
The new quiz is setup and you can find the boxes on the Gallios in Epalinges and in the main analysers room in the the Agora ! Please go fill up your answer and win the next bar !
|
|
|
|
FACS Tips
Seeing is Believing: Expand your Visions with the ImageStream
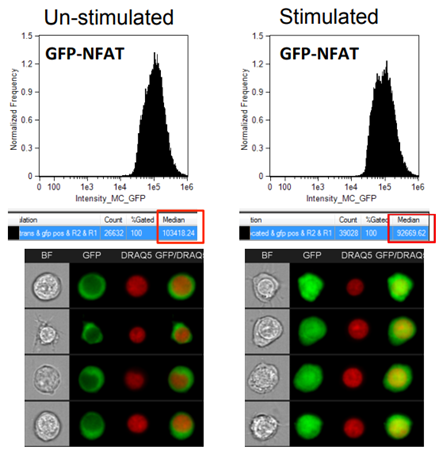
While we would never fault flow cytometry, it is our best and favourite research method, sometimes it does leave us wanting more. Take for example the situation below, by focusing only on the fluorescence intensity histograms we don’t get a full appreciation for the changes taking place. By introducing an image component, we can see a clear translocation of the GFP protein into the nucleus that was invisible by conventional cytometry. While it may be possible to move to fluorescent microscopy to attempt to answer some of these morphology-based questions, that can create challenges such as loosing the ability to analyze large numbers of cells necessary to properly quantify rare events and have statistical power. Thankfully in imaging cytometry we can find a compromise between these two methods to balance the high throughput nature of conventional cytometry with the imaging capabilities of microscopy.
|
|
|
Image from Cerveira, Joana et al. “An imaging flow cytometry-based approach to measuring the spatiotemporal calcium mobilisation in activated T cells.” Journal of immunological methods vol. 423 (2015): 120-30. doi:10.1016/j.jim.2015.04.030
|
|
|
Imaging Flow Cytometry
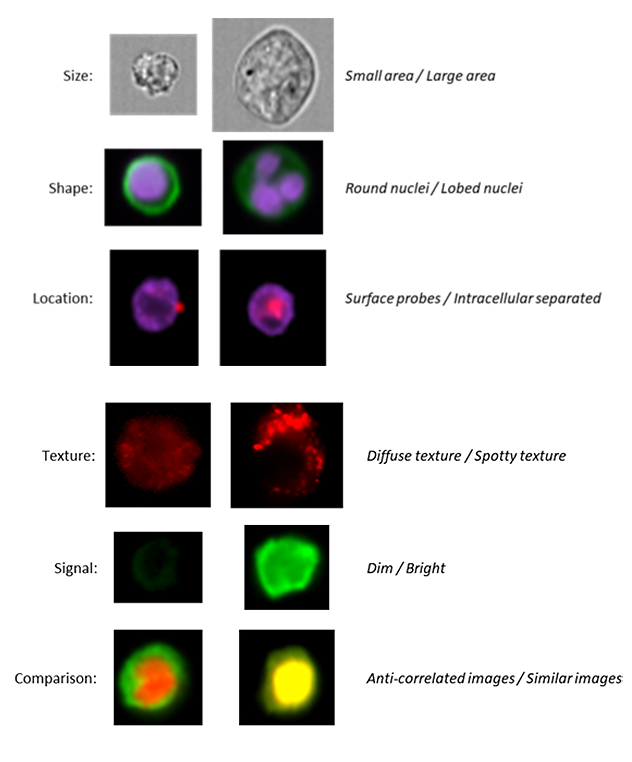
Imaging Flow Cytometry combines the strengths of Microscopy and Flow Cytometry in a single platform for quantitative image-based cellular analysis in large and heterogeneous samples. Essentially allowing you to get image-based information from the same experiment already being prepared for analysis on a conventional cytometer. So, while you can produce the traditional intensity-based plots you’re used to, image-based parameters are also measured from each cell, such as shape, texture, size, probe distribution heterogeneity, nuclear translocation, probe co-localization, subcellular localization of proteins, etc. Some of the applications that can be performed include:
|
|
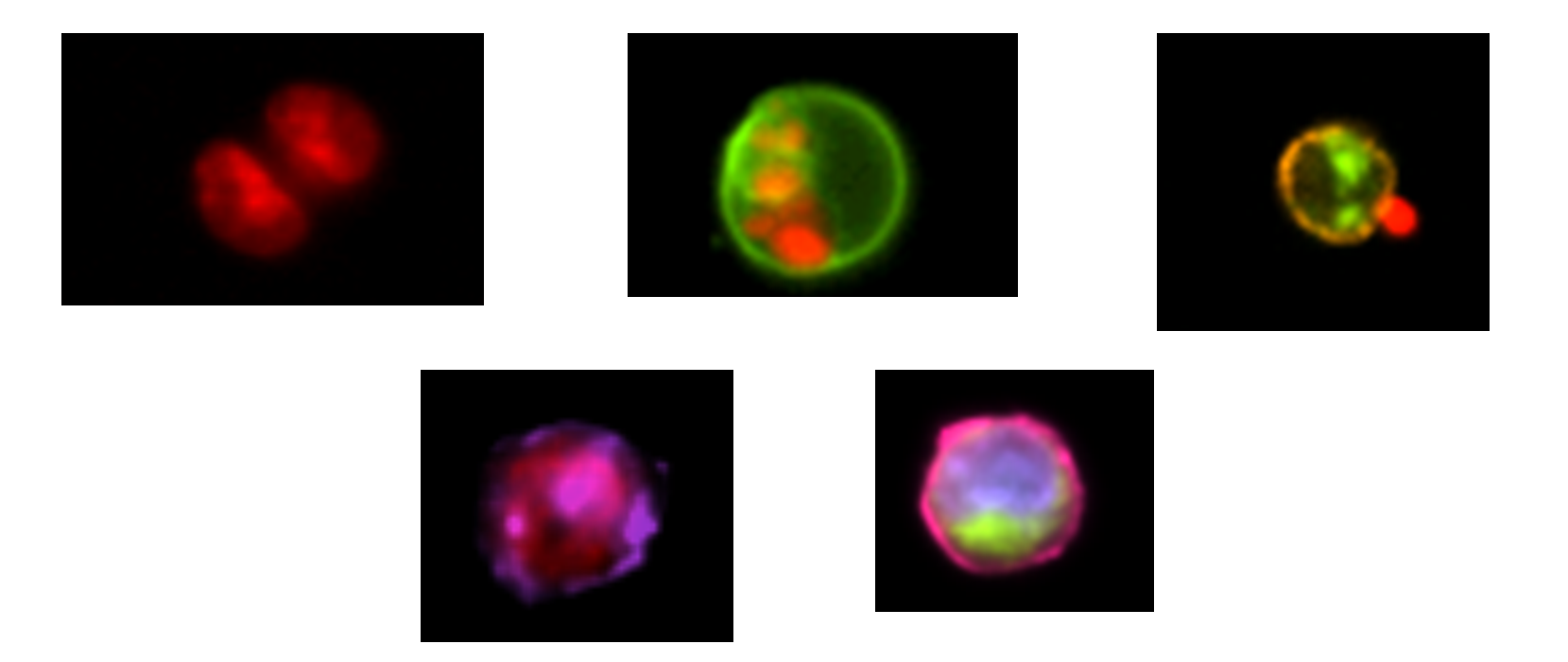
Co-localization of two proteins // Small particle analysis
|
|
Cell signaling // Nuclear translocation
|
|
Changes in morphology // Immune synapse formation
|
Parasitology // Autophagy
|
|
ImageStream
|
The ImageStreamX, located here at the FCF, is a high resolution, high speed, flow cytometer that numerically quantifies cellular morphology and the intensity and location of fluorescent probes at the rate of 1000 cells/second. The system is equipped with 20x, 40x and 60x magnification to accommodate a wide range of sample types ranging from nanoparticles to large cells. The instrument acquires up to 12 images of each cell comprising: a side-scatter (SSC) image, two transmitted light (brightfield) images and 9 fluorescence channels that collect your desired fluorophores’ emission from 430 to 800 nm. Images are collected on two specialized 6 channel digital cameras utilizing a novel velocity detection system to capture moving cells as still images. The result is high resolution imagery with fluorescence sensitivity superior to flow cytometry.
|
|
|
Preparation and Panel Considerations
|
|
|
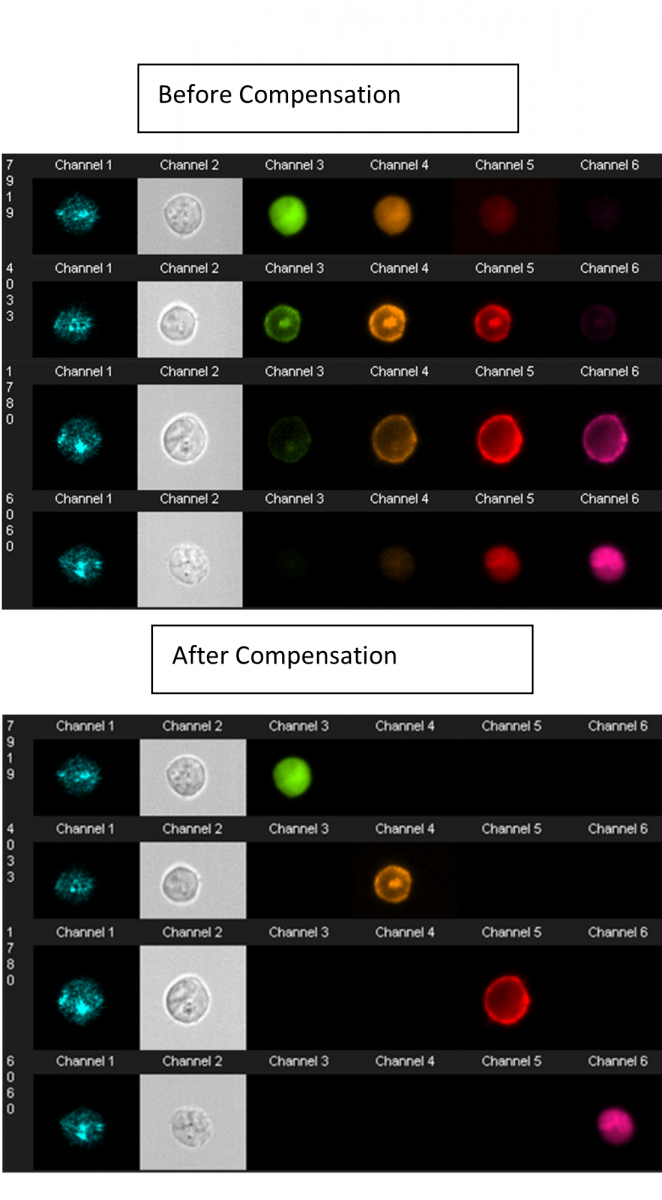
Most dyes used in conventional flow cytometry can be detected and compensation is still performed, but in a pixel-by-pixel and cell-by-cell basis, thus allowing for the use of more colours compared to microscopy. The system is equipped with 5 lasers (405nm, 488nm, 561 nm, 642nm and 785nm for SSC only) and 10 different fluorescent channels can be analyzed simultaneously. Data analysis is performed using IDEAS software, which allows the analysis of more than 90 parameters per single cell. It utilizes tools familiar to conventional flow cytometry analysis such as histograms and dot plots, while making morphological analysis readily accessible. As every cell is linked to a real-life image, by simply selecting a dot (on a dot plot) any cell can be visually evaluated. While ImageStream analysis can have a steep learning curve, it’s becoming more accessible through the addition of machine learning features allowing for more efficient use of the many parameters.
|
|
|
|
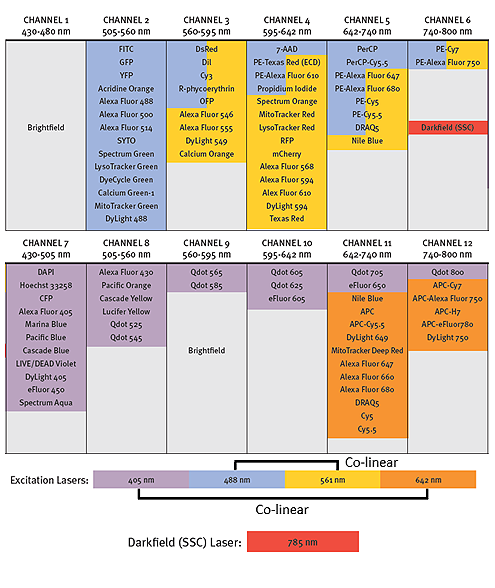
Above is the optical layout for our ImageStreamX instrument. Traditionally channels 1 and 9 are reserved for Brightfield images and the other channels show the typically used fluorochromes. Brightfield requires two channels, one for each camera to ensure they are displaying synchronized images. It is also possible to collect SSC in channels 6 or 12, but it is not always necessary to include depending on the experiment. As in conventional flow cytometry it is best practice to spread out your chosen fluorophores across several lasers. What’s more complicated about the ImageStream though, is that the lasers are arranged in co-linear arrays, with the 488nm and 561 nm, and 405nm and 642nm lasers paired respectively. This makes panel building harder as it is no longer possible to use the time delay to separate fluorochrome excitation off separate lasers, and fluorochromes excited by multiple lasers can create a more complicated spillover. For example, it is not possible to separate PerCp-Cy5.5 from PE-Cy5 by compensation, they both primarily collect in the same channel. So, while it may be possible to collect up to 10 fluorochromes, simplicity is certainly preferred on the ImageStream to get the best possible images.
|
|
Reasons to use imaging flow cytometry
- If you have a question that relies on knowing cell morphology
- If the spatial context of the signal within the cell is important to your research
- If you have rare cell events or short-lived events that are hard to find by normal microscopy
|
|
Conclusions
|
The ImageStream provides some exciting new experiment options and can be adapted from your existing experiments in flow cytometry. However, it is a more complicated process in both preparation and analysis to get right so it will require an investment of time and energy in these experiments to get them off the ground and achieve success. Feel free to reach out to the FCF staff if you would like to discuss the possibility of making an ImageStream test.
|
|
Can you answer this ?
|
|
|
|
|