|
|

|
|
|
|
|
Facility News
As the snow ❄️ and the health restrictions 😷 are melting away, I hope you are feeling good in this month of February !
|
|
I would like to announce that our beloved Danny is back at the Agora. He is the proud and happy father of Adam Friedrich Labes, born on January 16th ❤️. As a consequence, the sorting schedule starting 2050 and + has been opened since he will undoubtedly take over Daddy Danny's Legacy.
|
|

|
Danny is already back at the Agora with Mariela ! So the team is ready to help you in your projects. Additionnally, keep in mind that Kevin, Francisco and I are always available for sorts and trainings on either sites !
|
For this month FACS Tips, we are adressing the often asked question about compensation in DIVA vs Flowjo. Is there one better than the other or do they complement each other ? Find out !
|
Finally, the Royal Microscopical Society is running an interesting Flow Cytometry Data Analysis course from March 7th to March 10th. This course is aimed at those new to cytometry or those who want to expand their capabilities. They cover some of the common applications of flow - multicolour phenotyping (including compensation), cell proliferation (DNA cell cycyle and dye dilution), and funcional assays (apoptosis and calcium flux).
|
The course is supported by FlowJo and FCSExpress and delegates will have access to recorded tutorials as well as the data sets used in the course.
|
Places are limited to ensure maximum interaction. Don't miss this opportunity to learn from the very best cytometrist of the UK ! Registration and more details are HERE.
|
|
FACS Tips
Compensation in DIVA vs Compensation in FlowJo and a Look into the Future
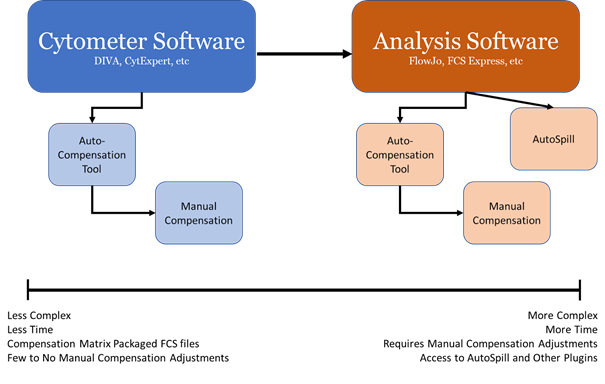
In the process of performing flow cytometry experiments you’ll notice that while we almost always calculate the compensation matrix on the cytometer using the automated compensation tool, we also have the ability to do this in our analysis software such as FlowJo. This can be confusing though, why have both, and when is it right to choose one over the other. On top of this, there is also the ability to manually create the compensation matrix on both the cytometer side and in the analysis software. While we wouldn’t recommend users to do manual compensation, it is occasionally valuable to make manual adjustments to the matrix since compensation isn’t always perfect and some slight over or under compensation needs to be corrected. Knowing the pros and cons of each of these features on both the cytometer and analysis side we can alter how we approach our experiments to get the best possible data.
|
|
|
|
|
Cytometer Software
When performing a flow cytometry experiment on one of the analyzers, your first opportunity to perform compensation comes in the collection software, be this DIVA on the BD machines, CytExpert on the Cytoflex, or SpectroFlo as part of the unmixing, analogous to compensation, on the Aurora. As trained here in the facility, we record our single stains, apply the compensation and then run our samples to get an accurate representation of our data, rather than running with no compensation applied. This gives us the ability to perform, on the spot, basic analysis of how our experiments performed. Additionally, when compensations are set and applied on the cytometer, the FCS files exported should be packaged with that matrix and bring that matrix with them into analysis software like FlowJo. This is not necessarily with a compensation matrix created in analysis software. So, if you’re in a situation where you need to share FCS files with multiple people, be aware that the compensation matrix used may not be packaged appropriately with the FCS files you are sharing. There may be extra steps that need to be done to accomplish this when a compensation matrix is created on analysis software.
|
Analysis Software
There are times where it does make more sense to apply, or reapply compensation afterwards in analysis software. It’s possible that we have a very large panel or very little time for our experiment, and have not optimized the gate placement of our single stains for compensation, but rather quickly put something together to record, with the expectation that we will fine tune afterwards. If your compensation matrix requires manual adjustments it can be a time-consuming process, and is often easier performed post acquisition on the analysis software as opposed to on the cytometer where time is billed. Most analysis software programs will auto generate n x n bivariate plots to compare every marker allowing much quicker and easier manual adjustments to achieve properly compensated plots than on something like DIVA. It is important to remember that manual compensation adjustments can introduce more error into the matrix unless done empirically, and if large adjustments are necessary to achieve this you may need to consider that there was an error in your experiment single stains and re-running signals may be necessary.
|
Compensation can be calculated and recalculated as much as you like, there is no limit. So, if you don’t feel confident you did the best possible compensation on the cytometer, you can redo it in the analysis software. One side should not be better than the other, as both make their compensation calculation from the same FCS files, the main changing variable between the two would be the placement of the gates.
|
Gate Placement
|
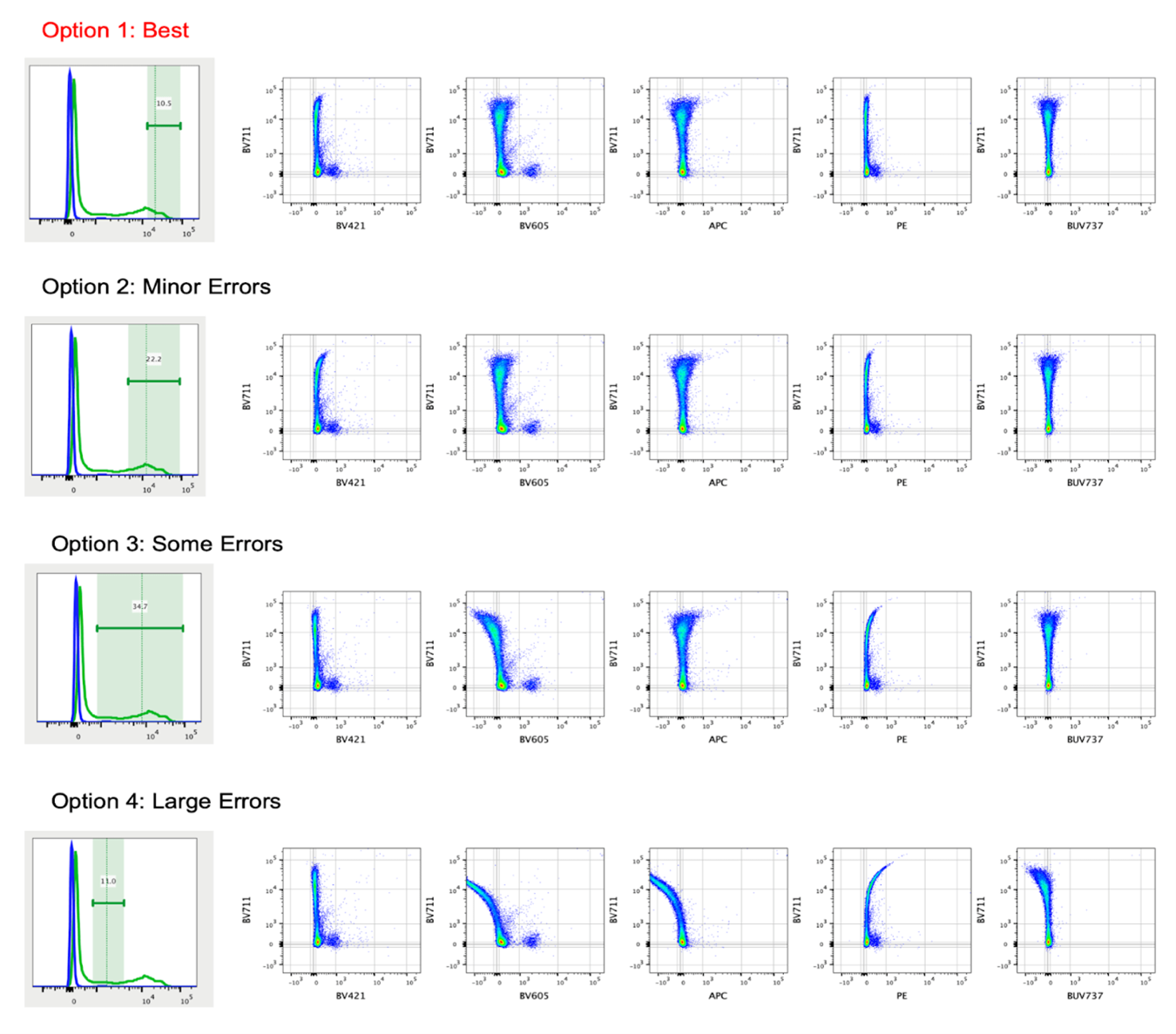
Gate placement in compensation is very important to reduce errors and get the most accurate matrix. When placing gates for both the positive and negative peaks, it is best advised to select the most positive cells, ideally from the center of the peak and higher in terms of fluorescent intensity, or center of peak and lower intensity for the negative. The reason for this relates back to the principles of compensation discussed in our June 2021 newsletter (link): positive cell population in our single stains should be as bright or brighter than the one in our fully stained samples, and this is best achieved by just gating the brighter portion of the peak for compensation calculations.
|
|
|
Image from https://voices.uchicago.edu/ucflow/2020/06/24/the-right-and-wrong-way-to-set-up-automated-compensation-tools-how-to-achieve-accurate-compensation/
|
AutoSpill and Algorithmic Compensation Calculation
|
Thanks to the introduction of AutoSpill software in FlowJo, it is possible to avoid gating altogether to calculate compensation. AutoSpill is an integrated compensation algorithm recently added to FlowJo that will automatically produce a compensation matrix, improving the estimation of the spillover coefficients. This allows for better compensation calculations even with very poorly defined positive and negative populations in your single stains. AutoSpill is even capable of removing autofluorescence when acquired properly with an unstained control.
|
|

|
|
As always feel free to ask the FCF staff if you need any help performing your compensation or making manual adjustments.
|
|
|
|
|
|
|