|
|

|
|
|
|
|
Facility News
Happy New Year 2022 🥳 ✨! We hope that this year will create great memories and bring you closer to your goals ! There is definitely a lot happening at the moment with the pandemic but rest assured that we will be there to support you !
|
We would like to remind our users that the best way to protect each other is to follow the rules on hygiene and physical distancing in all occasion. We will also enforce the rule of one user per machine. For Cytoflex users, once the machine is setup, it is encouraged to leave the room. You may also use TeamViewer to watch your run from the comfort of your desk. If you don't know how to use it, our team will gladly show you.
|
This month in FACS Tips, we are talking about Tandem Dyes and how to optimize their usage in your experiments !
|
Last reminder for today, Danny is not working for the month of January 2022 so if Agora users needs sorting or support, please refer to the Epalinges staff until further notice.
|
|
FACS Tips
The Highs and Lows of Tandem Dyes
In flow cytometry we constantly balance advances and advantages with challenges and increasing complexity, thus requiring more attention when it comes to our experiments. This is the case with tandem dyes. While their use massively expands the range of parameters we can analyze, allowing us to create double digit marker panels, they also introduce instability that can result in confusing data and a failing experiment. With companies racing to add more lasers and detectors to cytometers, the push to expand the catalogue of antibody fluorochrome conjugates necessary to fill these empty channels on more complicated machines follows swiftly. It is only more likely to see more tandem options flood the market to allow for greater expansion of our flow cytometry panels going forward, but with that comes challenges, and considerations that need to be made.
|
First developed in the late 1980s tandem dyes, as the name suggests, are two covalently attached fluorescent molecules. With one acting as the donor, undergoing the primary excitation, followed by a transfer of it’s energy to the acceptor, which then emits photons of light at it’s own wavelength. Even if it is a non-radiative energy transfer and not a photon exchange, the emission spectra of the donor must overlap the absorption spectra of the acceptor (except for the Brillant Violet dyes). This process, known as fluorescence resonance energy transfer or Förster resonance energy transfer (FRET) allows two closely linked fluorophores (roughly 1-10nm apart) to behave as a unique fluorophore.
|
|
Tandem dyes are composed of either a protein based fluorescent molecule, like PE or APC or a synthetic, polymer such as BV421, that have a large capacity to absorb energy and are attached to a smaller synthetic dye that serves as the acceptor, like Cy5. The brilliant violets are especially interesting as they do not require the emission of the donor to overlap with the excitation of the acceptor as in conventional PE or APC based tandems. This makes it possible for a higher stoke shift, the difference between excitation and emission maxima, and far emission range like 785nm compared to the initial excitation at 405nm.
|
|
|
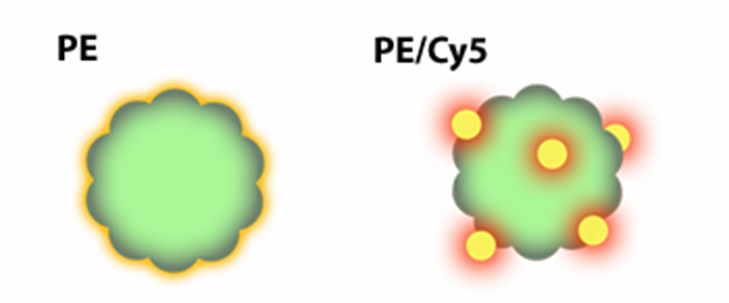
PE-Cy5 tandem dye. Both molecules are excited by the blue or YG lasers, based on the excitation properties of PE. Image from https://www.biolegend.com/en-us/tandem-dyes %20Protein%20(PerCP)%2DCy5.
|
|
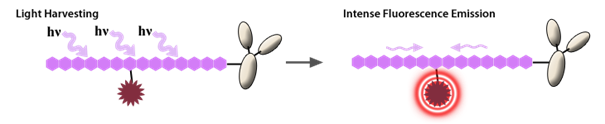
Example of a Brilliant Violet tandem dye. Image from https://www.biolegend.com/en-us/tandem-dyes
|
|
|
A source of concern for tandem dyes is the potential for degradation, the separation of the acceptor from the donor. It’s important to note that FRET is not a 100% efficient process, so there should always be some expected fluorescence in the donor channel, for example from the PE in a PE-Cy7 tandem. This itself is not a sign of degradation. Due to a covalent bond, it is also uncommon for donor and acceptor to separate in solution. However, if it becomes a strong signal in the donor channel and a weak signal in the expected channel, this is a symptom of a problem with your antibody. If your experiment includes both PE and PE-Cy7 and the tandem undergoes degradation, there will be false positive staining in the PE channel creating an appearance of under-compensation that cannot be corrected. The final effect is that your PE stain will now more likely include false negatives. There are several possible explanations for tandem degradation listed below;
|
- Photobleaching - Tandem antibodies are particularly vulnerable to oxidative damage from light exposure, make sure to protect samples from light as best possible.
- Exposure to freezing temperature - This can result in denaturation of the protein fluorophore. It is still important to stain on ice, however avoid freezing any tandem antibodies.
- Sample incubation – When working with unfixed cells, it is possible to have cell mediated uncoupling of the donor and acceptor, this is especially true of APC based tandem dyes. This is another reason why it is important to stain samples on ice to slow down cell metabolism.
- Fixation and permeabilization - Leaving antibodies in fixatives for too long can increase the amount of autofluorescence and also increase instability of tandems. 10 minutes in fixation is normally sufficient.
- Shelf life – As mentioned previously tandem dyes are less stable and more prone to degradation over time and it is important to pay attention to the expiry date on the tube.
Another concern of tandem dyes is inconsistency, this can be seen between different suppliers, and even between lots from the same supplier. There can be differences in the ratio of donor to acceptor molecules, and in the ratio of fluorochrome molecules conjugated per primary antibody, producing different emission spectra and intensity between lots. The tandem dye used in an experiment should be from the same tube as that used for the single stain to avoid inaccuracies in compensation controls.
|
|
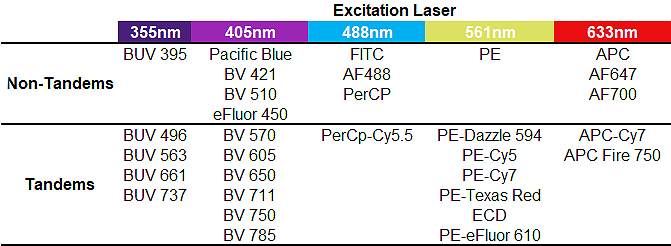
Tandem dyes are an increasingly necessary part of our flow experiments, allowing us to build bigger panels and assess more parameters. They come at a cost though as they introduce more unpredictability if they are not used appropriately. It is important to rerun compensation controls frequently, especially when moving from one tube of a tandem dyes conjugates to another to control for any differences. It is also useful to always look for unusually high expression in the donor channel of your tandem signal stains for signs of degradation. Improved production of tandems has made them increasingly more stable and consistent between lots, however they will still require more care than primary fluorochrome antibodies. Feel free to ask us any questions you have about tandems or how they may influence your panel. Below is a non-exhaustive list of some tandems and non-tandems. While some are easy to spot because of their dual naming system, others less so, so it’s important to not what fluorophores you’re working with.
|
|
|
|
|
|
|
|