|
|

|
|
|
|
|
Facility News
Merry Christmas & Happy New Year in advance 💖
|
This month in FACS Tips, we explain one of the mysteries of life...in a Flow Facility - the difference between the photon detectors on the Cytoflex and those on the LSRIIs. It might be what you need to know to give a chance to our Cytoflex family ! Especially now that we have the Cytoflex LX3 with 6 lasers to work with !
|
We would also like to welcome Mariela Castelblanco as the new flow cytometry operator at the Agora. She will be working at 50% for the first six months and then full time thereafter. We are very happy to have some more help at the Agora to make sure we can always support your research !
|
As in previous years the Flow Cytometry Facility (Biopole 3 and Agora/CHUV labs) will be officially closed from 6.00pm Thursday December 23rd 2021 through to 12.00 noon on Monday 3rd January 2022. During this time there will be no FCF support or training, and no possibility to organize repairs as the service engineers from all companies are also on vacation until January 3rd 2022.
|
|
FACS Tips
Why I can change my gains after compensation on the Cytoflex, but not my voltages on the Fortessa !
In learning to perform flow cytometry on BD machines, the Canto, LSRII, or Fortessa, one of the essential rules we learn is that it is not possible to change the voltages after recording our compensation. Once the voltage is changed it invalidates our compensation matrix, and forces us to either rerun the single stains at the new voltage settings or work without compensation. This can be a frustrating roadblock when trying to move efficiently through experiments and requires more reagents for compensation. It can also be quite confusing moving experiments to the Cytoflex, where this rule no longer applies, and voltages or gains can be changed at any point without error.
|
|
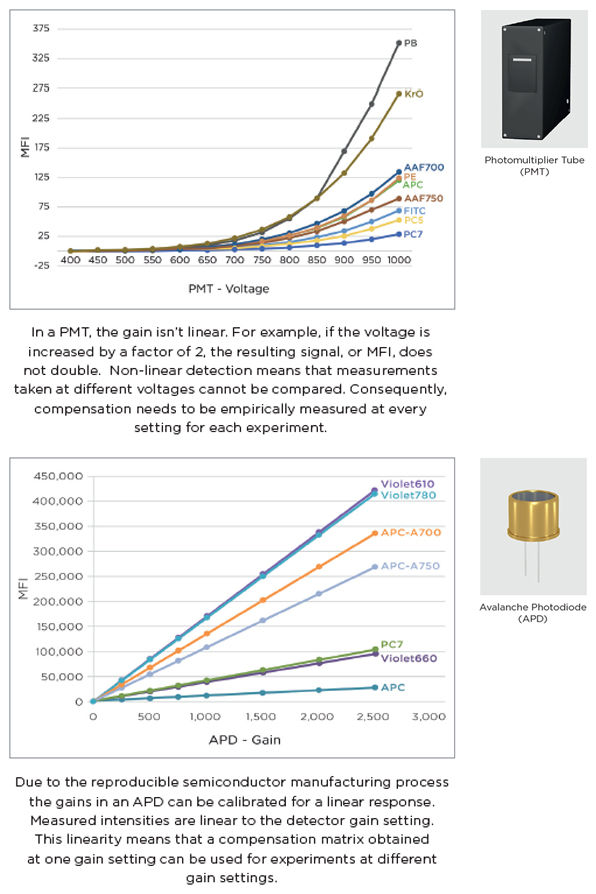
This is because the voltages are not linear on most classical cytometers, including the BD machines that we’re most familiar with (listed above). This is a product of the photon detectors installed on the machine. Fluorescence signals themselves are quite weak, and require amplification to be measured. The voltage adjusts the level of amplification, and this is done by Photomultiplier Tubes or PMTs (Top). When we increase the voltage of a detector on a PMT equipped machine, we not only amplify the signal of the fluorophore we are interested in, but also every other fluorophore that spills over into that detector. In addition, any increase in voltage isn’t necessarily equal within the same detector. For example, a 10 voltage point increase from 450 to 460 and 550 to 560 for a detector will not necessarily mean an equivalent increase in MFI, that’s why the system is considered non-linear. For this reason, if we change the voltages, we have not only changed the measurement for just one fluorophore, but also that of every fluorophore that spills into that detector in an inconsistent way. The compensation matrix is calculated from this measurement and therefore the voltage values must be fixed.
|
|
|
Adapted from https://www.mybeckman.uk/flow-cytometry/instruments/dxflex/high-complexity-flow/gain-independent-compensation
|
The Cytoflex however has Avalanche Photodiodes or APDs (Bottom) instead of PMTs, and these detectors produce a linear relationship between the gain setting and the MFI. Since the relationship is linear, it becomes possible to recalculate compensation matrix values after a gain value change on an existing experiment. This means that you can start an experiment with one gain setting, and switch to a different setting later in the experiment, or in a future experiment without removing the compensation or producing error messages. This system is called Gain Independent Compensation. This advancement in technology is excellent, especially for inexperienced users as it’s easier to correct mistakes and make adjustments without having to repeat compensation and waste time. It is also part of the system on the Cytoflex that allows the flexibility to save previously run single stains in the compensation library and mix and match them with new experiments.
|
|
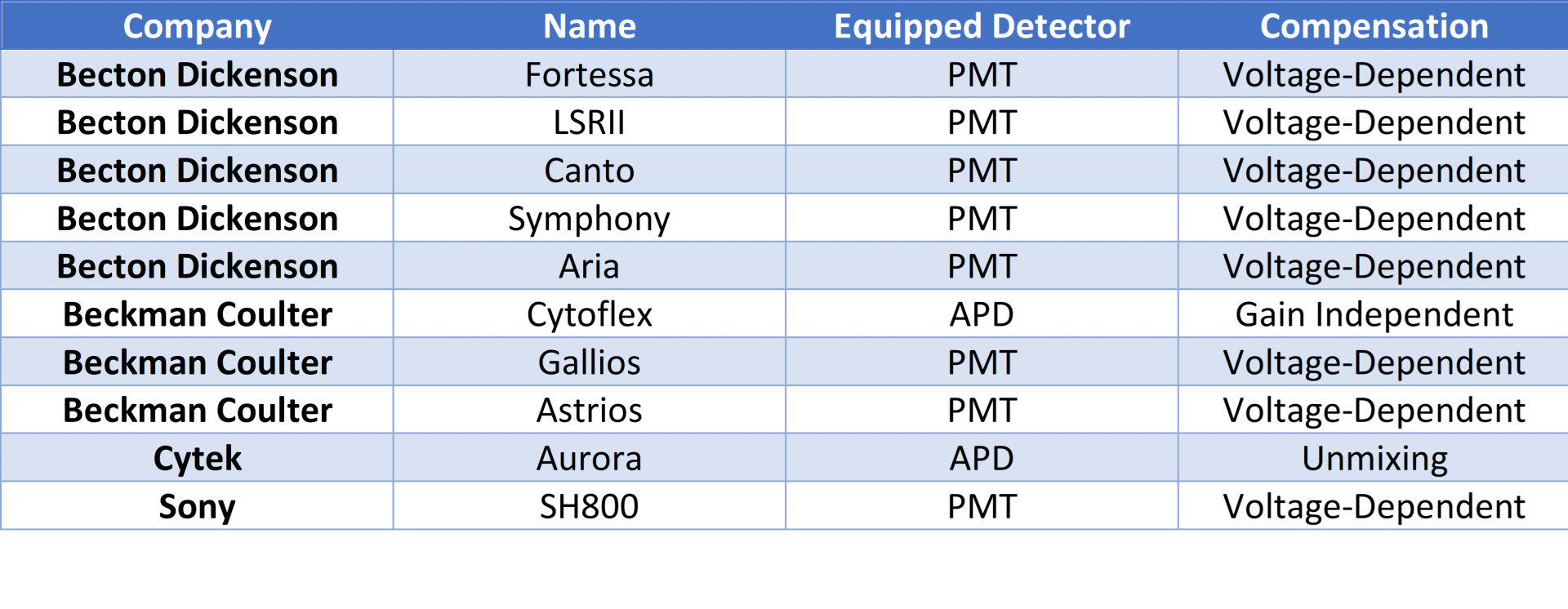
This is one of the attractive features to consider if moving your experiment to the Cytoflex. Especially if your experiment contains fluorescent proteins that have inconsistent fluorescent intensities, so you can ensure they stay on scale. While this tool can be very useful, it’s important to not cut corners in experiment preparation. It is still necessary to follow correct compensation steps, and it is essential to rerun new compensation samples for any tandem dyes in your experiment. Below is a table of machines available at the FCF and their equipped detectors.
|
|
|
|
|
|
|
|