|
|

|
|
|
Facility News
Hello all ! November is already on its way and it is time to stay home, enjoy some hot cocoa and relax watching the newest, hum, online seminar of course 😉
|
This month in FACS Tips, we have a big topic at hand ! We will talk about The Rules of Panel Design and make sure you never get terrified again at the idea of making a 10 color panel that your PI is asking for immediately ! Those rules works for both traditional and spectral flow cytometry, so it is universally useful !
|
We are also very happy to welcome Jean-François Mayol as the new head of the FCF Facility. After working for more than 10 years in Biotech R&D in France and Switzerland, he's decided to come back to academia and use his skills to help us provide the best flow cytometry services we can !
|
|
|
|
FACS Tips
The Rules of Panel Design
One of the most daunting challenges a researcher has in flow cytometry is designing functional staining panels. With the ever increasing number of measurable parameters, the wide choice of antibody/fluorochrome conjugates and machine diversity, it can be very intimidating to even tackle the task. Yet, by following a few simple rules (applicable to both conventional and spectral flow cytometry), we can improve our existing panels and make even better ones going forward !
|
Clarify your Objective(s)
It is important to think about the biology of your model (cell type, in vivo or in vitro,...) and the analysis you want to perform (phenotyping, cytokine secretion, phospho-flow...). It will guide you to a clear question to answer and prevent you adding too many parameters that could be counterproductive for the quality of your data. Once you know the main goal of your project, we strongly suggest consulting the Optimized Multicolor Immunofluorescence Panel (OMIP) published in the Cytometry A journal. These peer-reviewed panels are readily available online and have been created and verified by experts in the field. There is only so much you can do when always looking at the same markers on similar machines. Save yourself some time and use the OMIP as it is or as a backbone for your project. You will be glad to save some precious time !
|
Choose your Cytometer
You have decided the markers you want to measure and you now have a clear idea of the type of flow cytometry application you want to perform. It is a good time to decide which machine you will work with. It will dictate the maximum number of channels available to you among other things. Each machine has its strengths and weaknesses and you should use this to your advantage. This decision will not only impact upon the resolution of your data but also the practicality of it. Not every machine has a UV laser so keep that in mind when planning to create a backbone panel that could be further expanded in subsequent experiments.
|
Define your Gating Strategy
Surprisingly, the next step is to go to the end goal of your experiment, your results figure. Indeed, it is really useful and revealing to sketch the gating strategy and highlight the plots that will ideally be shown in your paper. It will help you define the relationship between different markers such as co-expression or mutually exclusive patterns. It will also remind you of the expected intensity of each fluorophore.
|
|
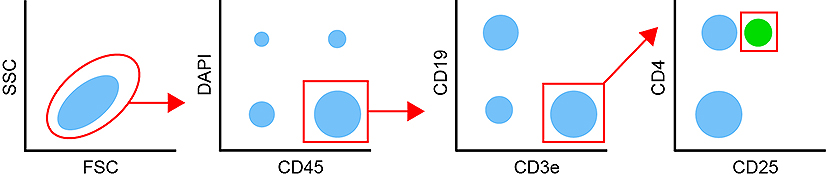
|
In this example, working on mouse splenocytes, we plan on gating on CD45 + DAPI – cells and plot CD19 + (B Cells) against CD3e + (T Cells). Those last two markers are mutually exclusive so it would be ok to use fluorochromes that tend to heavily spill over into each other’s channel e.g. BV605 and BV650. On the contrary, the next step is looking for CD4+CD25+ T cells that represent regulatory T cells, our target population. The two markers are co-expressed and CD25 is a low expression antigen. In that context, you want to use the brightest fluorochromes available to you that don’t spill over in each other’s channel. PE, BV421 or APCwould be a premium choice. The use of non-tandem dyes will also ensure a lower variability for repeated experiments.
|
Evaluate your marker patterns
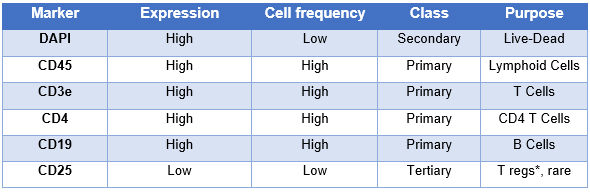
Once you know the markers and strategy, it is useful to define the expression patterns of your markers as well as their role in your question. The appropriate way of doing so is to break them down into three groups :
|
- Primary Markers : These markers have a well characterized expression pattern and are usually easily defined as either positive or negative. They tend to define your broad populations like T cells or B cells, etc... CD4 and CD8 in splenocytes would be a good example.
- Secondary Markers : Markers in this group will define sub-populations and can often have a mid-range expression or a continuum of expression. You will use these markers to define your naive T cells or memory T cells. CD44 would be a good example in this group.
- Tertiary Markers : Finally, these markers are usually what your experiment relies on. They have a weak or unknown expression pattern. They could also define rare populations. A T cell population with rare occurrence identified with a tetramer or PD-1 expression levels are good examples of these markers.
Fluorochrome Staining Index (SI) and Spillover Spreading Matrix (SSM)
Based on the machine you’ve selected, you can have a pretty good idea of the fluorochromes available to you. You can check your lab stocks and available antibodies in suppliers online and put together a table with the options available to you.
|
The first aspect to consider would be the SI of your dyes. As we have seen in a previous newsletter, every dye is different in its ability to emit light and it is important to know which fluorochromes are the brightest on the machine you’ve selected.
|
|
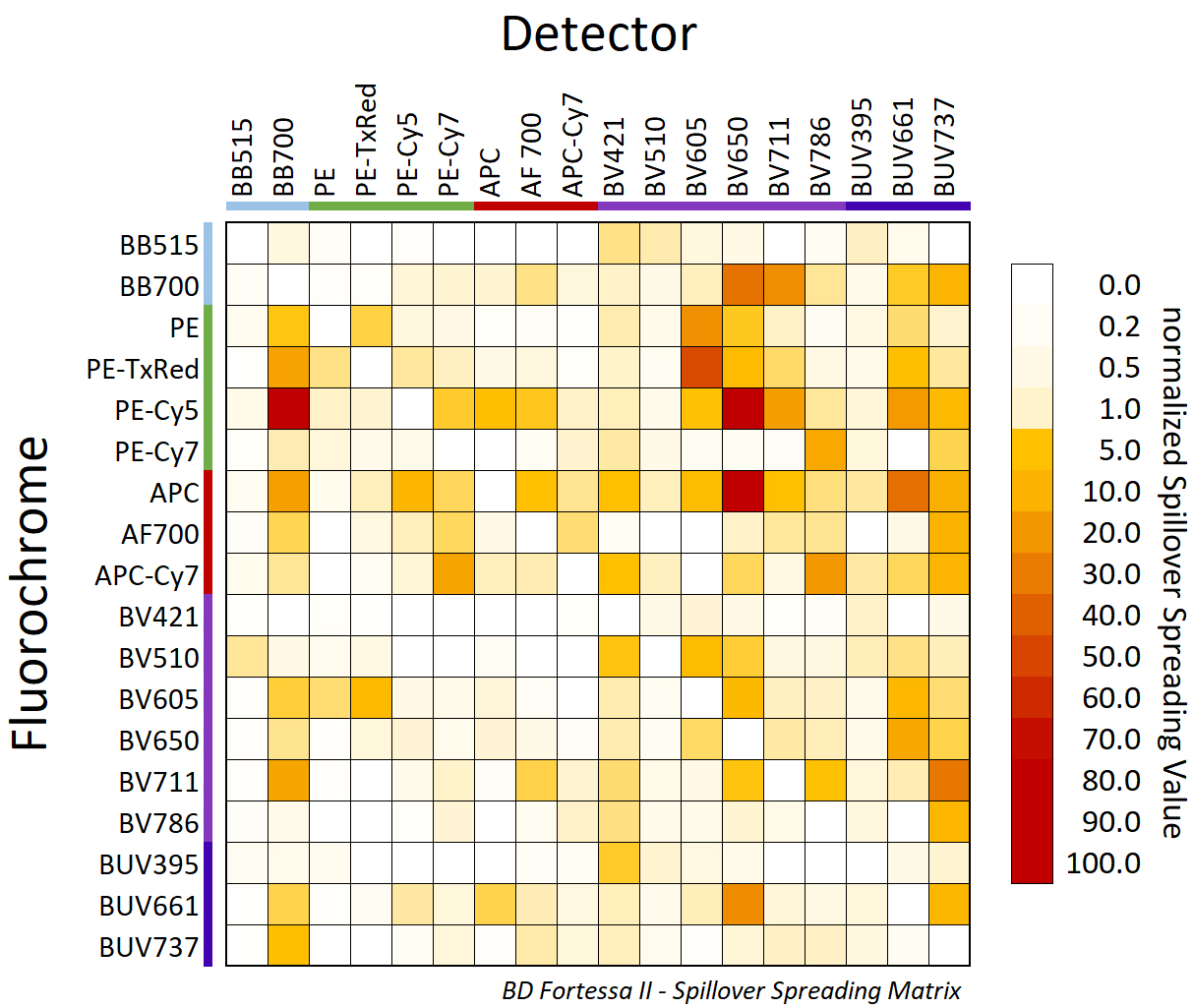
Secondly, you should take some time to check the spillover behavior of each fluorochrome using the SSM. The SSM summarizes how fluorochromes behave together by measuring the amount of signal spread from one fluorochrome into the other channels detectors of a given machine. It can be quickly produced using single stain in each channel and run on the machine of interest. The color in column Y spread in to the channels of column X, he higher the number the higher the spread. As you can see below on our BD Fortessa II (Agora), PE-Cy5 would be spreading quite a lot in multiple channels (APC, BB700, BV650, BUV661) which is why it is a color we usually discourage to use in large panels.
|
|
|
Keep in mind that buying new antibodies might seem more costly at first but could end up more productive in the long run.
|
Create the Panel Table and choose your pairings
|
With all the gathered information, it is possible to create a detailed table that will guide you through the creation of your panel (see below).
|
|
|
To choose your pairings and make your decisions, you should think about the following rules :
|
- Low expression markers (tertiary) should be paired with most bright fluorochromes and preferably with non-tandem dyes
- High expression markers (primary) should be paired with fluorochromes having a lower staining index.
- Do not pair co-expressed markers with fluorochromes that spillover heavily into their respective channels.
- Conversely, mutually exclusive markers can be paired regardless of the spillover status of two channels.
- Spread the color choices across all available lasers (don’t use all BVs for example).
- Spread the color choices through the spectrum on each laser line.
|
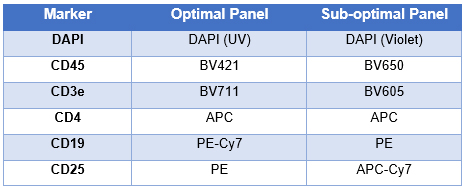
If we take the previous panel, a good panel option for the 5 lasers Fortessa in our facility would be :
|
|
|
We are making sure to separate correctly CD4+CD25- and CD4+CD25+ population, our target of interest. The colors are spread over the lasers or through the spectrum of each laser line, reducing compensation and spillover spreading. The sub-optimal panel would have trouble discriminating CD45+/- and CD3e+/- as it uses BV605 and BV650, two fluorophores known for high spillover. Additionally, we are not using the blue laser and therefore not benefiting from the full potential of our machine.
|
Panel Evaluation with the FCF Team
|
At this stage, we would be happy to look at your panel and evaluate it before you start testing it out or buying expensive conjugates. Bring all the information and let’s discuss it together.
|
|
|
|
|
|
|