|
|

|
|
|
Facility News
Hello all ! Yeah, fall is upon us. Not like it was different from our summer. At least, the kids are back to school ! I hope you feel rejuvenated and ready to work with us again !
|
This month, we talk about the ues of dead cell exclusion dyes. It is often an afterthought (if thought about at all) in people's panel design. Yet, it could simplify the struggle of identifying the mysterious new population your PI is excited about (You know, that All Markers+ population that makes no sense...). So please take a look at our suggestions and always include a dead cell marker in your panels !
|
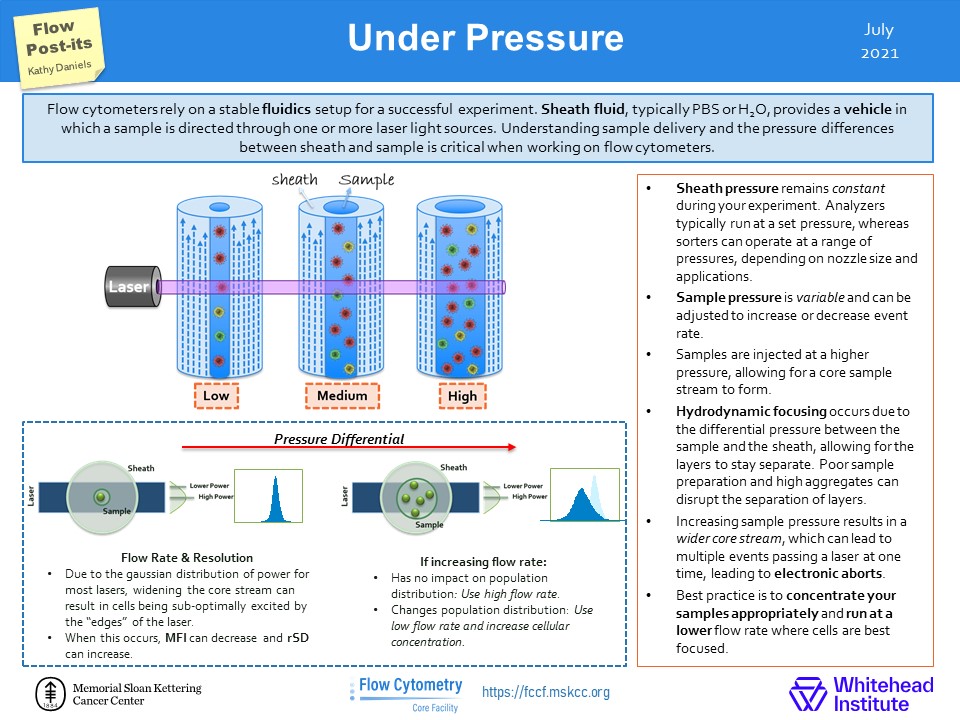
In the Flow Post-it ! We explain the consequence of changing the fluidics pressure while running your samples. It might seem cool to run your samples at high speed in an hour but is it worth the data spread you end up having ? Please check it out !
|
Finally, I would like to mention that the FCF Team is now able to train users on FCSExpress. FCSExpress can be a great alternative to Flowjo. It provides a fully integrated excel and graphics tool alongside conventional flow data analysis. It is more focused on batch analysis and can update in real time your statistics as you adjust your gates. Contact us if you are flow-curious about it !
|
|
|
|
FACS Tips
Dead Cell Exclusion
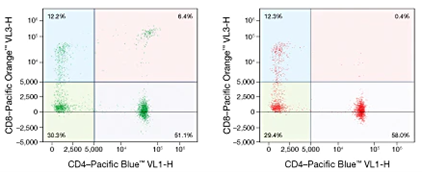
Dead cells can have a profound effect on our data, and a CD4/CD8 double positive peripheral population is one of the clearest examples of this. Dead cells take up or bind antibodies non-specifically creating populations and events that should not be there. An otherwise good experiment can be ruined by plots filled with erroneous populations, or “new cell types” that just don’t exist. With the assistance of a cell viability dye, dead cells can be excluded from our analysis and thus ensure we’re looking at the right thing.
|
|
|
From Thermo Fisher https://www.thermofisher.com/ch/en/home/references/newsletters-and-journals/bioprobes-journal-of-cell-biology-applications/bioprobes-issues-2011/bioprobes-64-april-2011/eliminate-dead-cells-for-increased-accuracy-april-2011.html
|
|
|
The process of removing dead cells begins just by distinguishing cell size. Dead cells tend to be much smaller in terms of forward scatter (FSC) than live cells, and by ensuring we don’t push our FSC/SSC gate too far to the left we can already exclude some dead cells. This won’t completely solve our problem though, and this is where cell viability dyes are required. There are plenty of options when it comes to viability dyes, however picking the one that fits with your experiment goals takes some thought.
|
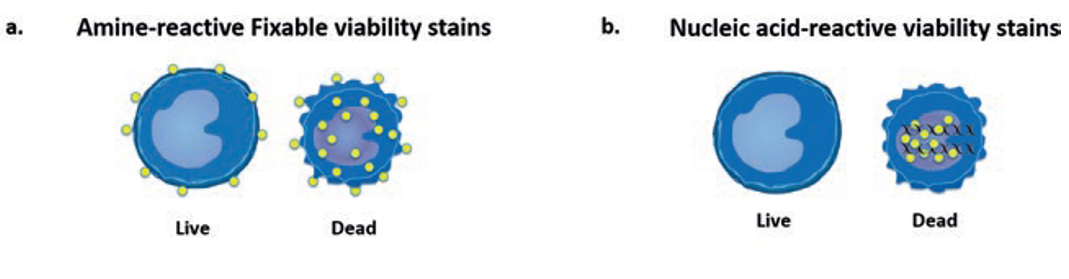
Cell viability dyes come in two main types, cell impermeant DNA binding dyes such as DAPI, 7AAD and PI (b), or dyes that label proteins like the Live/Dead fixable viability stains (a). The decision as to which one to choose depends on your staining panel or how your sample is prepared. For example, DAPI is a great viability stain for unfixed cells or for sorting purposes because it provides you with a live monitoring of cell death. However, it means you cannot use BV421 or Pacific Blue in your panel if the machine does not have a UV laser. DAPI is not fixable though, and any discrimination of dead cells with DAPI if your sample requires fixation is impossible. In contrast, Thermo Fisher’s line of Live/Dead fixable dyes, like Aqua or Near-IR, are preserved even through fixation due to the covalent bond between the dye and the protein of interest. For intracellular staining experiments, these amine-binding fixable dyes are required and are flexible in channel/laser choice as they come in a wide range of colors. If your experiment requires fixation, it is very important to make sure beforehand if your chosen viability dye is fixable.
|
There are also permeable dyes that will positively stain the DNA of live cells, such as Hoechst or DRAQ5. Occasionally it is of value to combine a permeable and non-permeable viability/DNA dye in the same experiment, such is the case in sorting when dealing with a sample with lots of debris or myelin, or determining viability in bacterial cells with the combined use of PI with membrane-permeable DNA-binding counterstain.
|
|
|
Goetz, C., Hammerbeck, C., & Bonnevier, J. (2018). Flow Cytometry Basics for the Non-Expert [electronic resource] (1st ed. 2018.). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-98071-3
|
Conclusions
|
One of the easiest ways to improve the quality of our data is to include a viability dye. It ensures that non-specifically bound dead cells can’t interfere with the expected population spread of our experiments. This can have important downstream affects especially in sorting when selecting cell populations for experiments like RNA sequencing where these dead cells cause contamination. While it may require some trial and error to introduce the correct dye into your specific experiment, it provides insurance of properly collected data.
|
|
|
|
Flow Post-its
Social media is a great source of information even when it comes to flow cytometry. One useful resource is the twitter account of the Flow Cytometry Core Facility at Memorial Sloan Kettering Cancer Center (https://twitter.com/FlowMskcc). We thought it would be nice to highlight their tips and bring them to you !
|
|
|

|
|
|
|
|
|