|
|

|
|
|
|
|
Facility News
|
As August settles in, the Paris Olympics 2024 🏃🏊 are going full force and this week offers many exciting finales to watch ! I hope you are enjoying the show remotely or in Paris ! Did you know that the Olympics were not always a sport only competition ? In the first editions, there was also art competitions across different categories: architecture, literature, music, painting, and sculpture ! Why don't we try to bring back a bit of diversity and add Science Competitions to the mix ? Who wants to win the gold medal 🥇 in Challenging Transduction, race for the highest number of I.V. injection per hour or the number of cells sorted from a complex tumor prep within a working day ? Something to keep you entertained as you are running your flow 😉
|
|
In this month FACS Tips, we're going a bit technical ! Kevin is describing what is the threshold parameter and how it can be used efficiently in your experiments.
|
|
Abigäelle Pelletier won the mug this month, Congratulations to you !
|
|
Each month, we will ask you 3 questions about the newsletter topic. If you win, you can enter the lottery to win a unique mug designed by the FCF team !
Please take few minutes to answer the quiz HERE.
|
|

|
|
|
FACS Tips
|
Threshold and What's Behind the Invisible Wall
|
|
Despite its crucial role, the threshold of an instrument is often an overlooked parameter in flow cytometry experiments. It sets the boundary between what we consider an event of interest and what we consider background noise to be ignored. Essentially, it is the electronic hurdle that must be cleared for an event to be recorded by the cytometer. It’s quite possible you can carry out experiments on a cytometer for years having never adjusted it from its machine default.
|
|
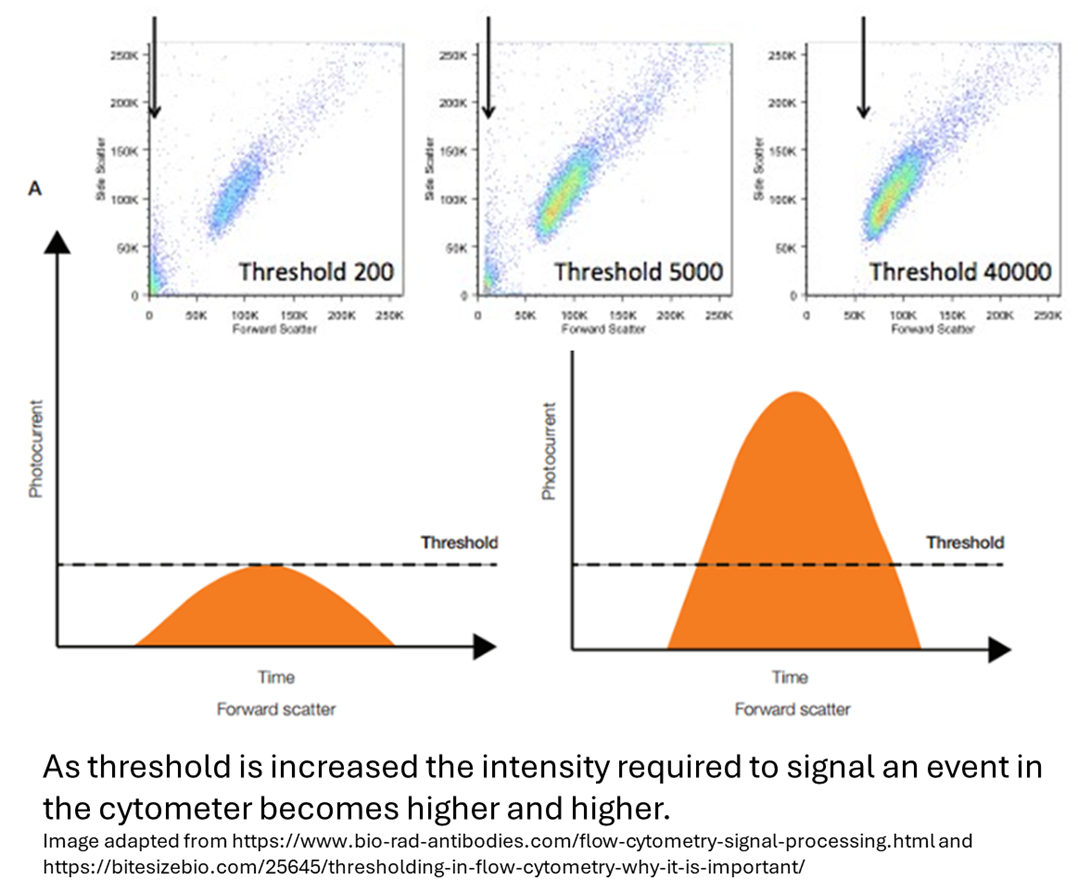
|
|
The default threshold is typically set based on Forward Scatter (FSC) and is usually applied at about 5% of the instrument's dynamic range; on BD cytometers, this corresponds to a value of 5,000. Adjusting the threshold can exclude debris in messy samples by increasing it, or it can help analyze small particles by decreasing it. You can even change the threshold to other parameter channels or combine a parameter channel with the FSC. A useful example would be trying to investigate TILs in a very messy sample. If the threshold is changed to a carefully set value off say a CD45 marker, then this can clean your immune cells out of the noise. This works great on the analyzers, however, it’s not advisable while sorting. This is because the machine is only blinded to the debris, so you may not see it in DIVA, but it’s still in the sample and can contaminate the purity of the sort. While this parameter seems straightforward, the threshold can both cause and resolve some unusual flow anomalies.
|
|
Baseline Restoration
If you've ever encountered an error where a negative population adjusts well below zero while running a sample on a BD instrument, it could be due to a feature called Baseline Restoration. The cytometer continuously introduces unwanted background noise into the measured signal; however, this signal is typically very small and falls below the machine's threshold. The software averages this background signal and subtracts it to prevent interference with the signals of interest. This process occurs in every detector and channel.
|
|
This does have the potential to produce problems, particularly with unbound antibodies. Since the threshold is usually set based on FSC, unbound fluorophores are often too small to meet the detection threshold but will still emit fluorescence when passing through the laser light. This emitted light becomes part of the background subtraction in that channel. Small amounts of unbound fluorophore may go undetected, but a large amount can cause a persistent fluctuating negative signal below zero. This issue is especially noticeable in single stains performed immediately before running, without first washing out unbound fluorophores. A similar issue can occur with DAPI bound to free DNA in samples, as I will demonstrate below.
|
|

|
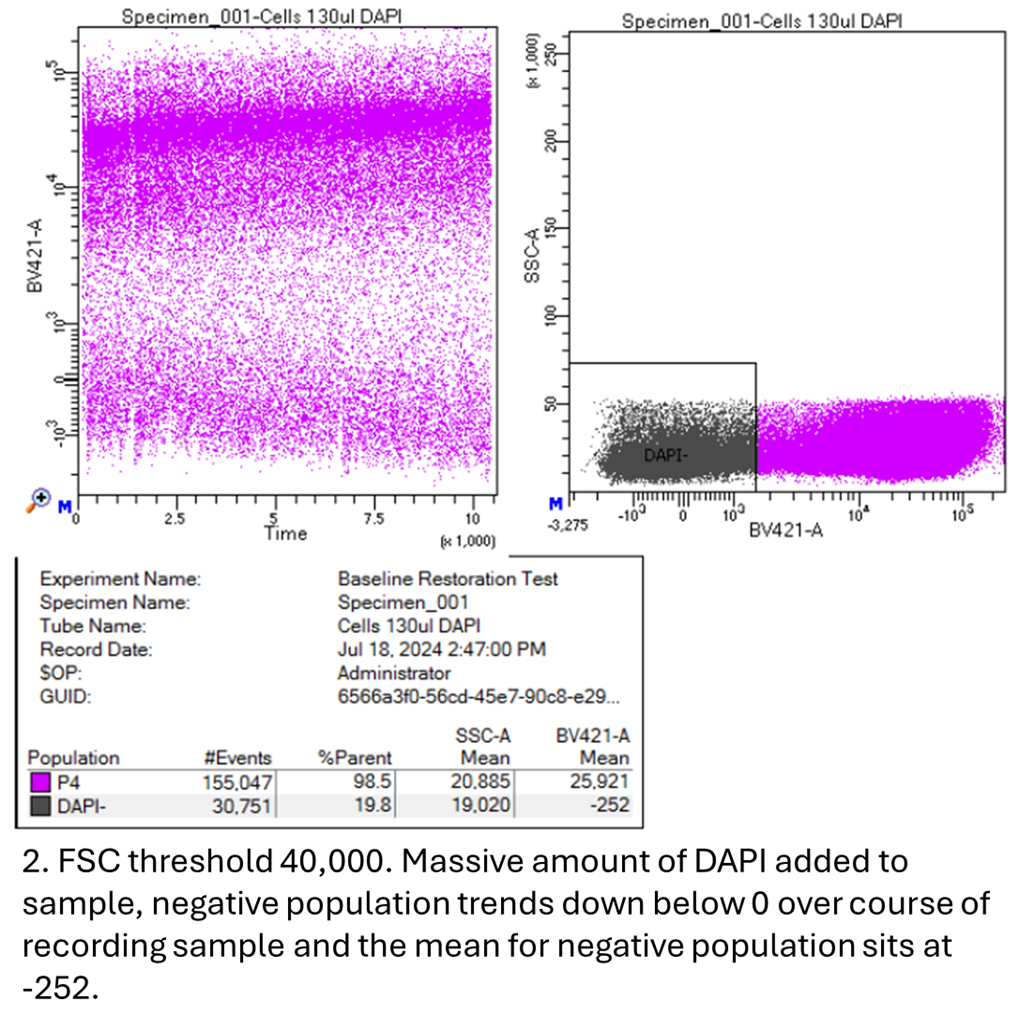
|
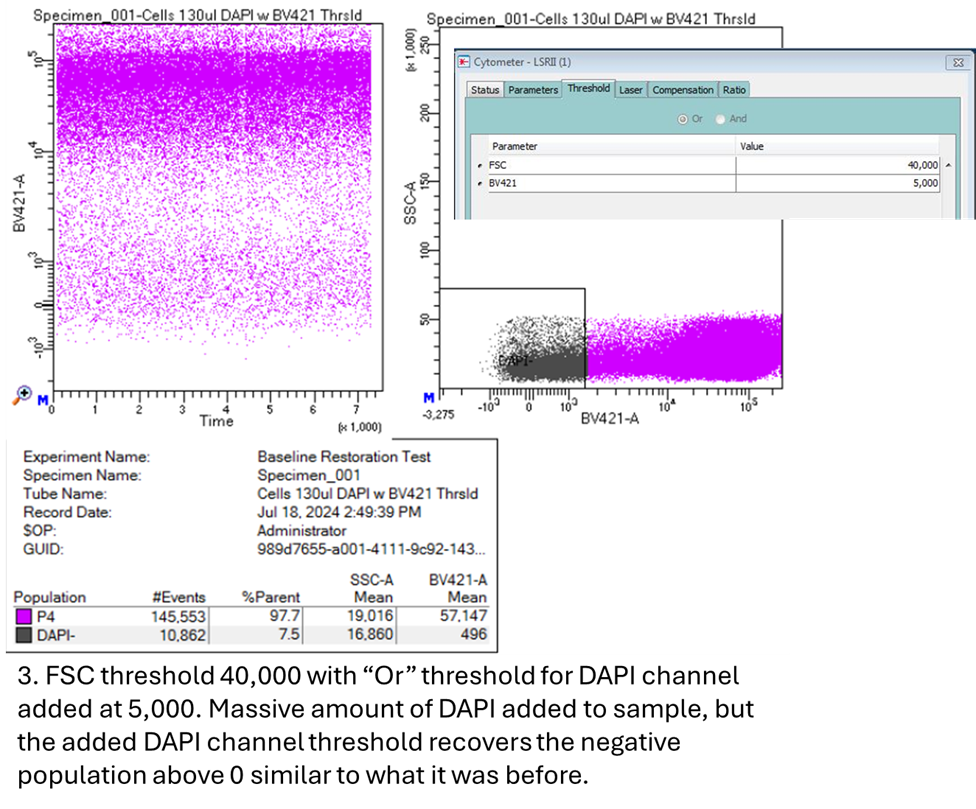
|
Using the LSRII, I adjusted the threshold to a higher-than-normal value of 40,000 based on FSC. When staining a cell sample with a normal amount of DAPI, the mean of the negative cell population appropriately sits above 0 on the DAPI biexponential plot. However, when I add an excessive amount of DAPI to the sample, saturating any potential free DNA or dead cells beneath the high FSC threshold I've set, the negative shifts below 0. Over time, this population becomes increasingly negative as the sample runs.
|
There is a solution to this though, we can add a second threshold in the channel that is adjusting beneath zero. In my example, introducing an “Or” threshold in the DAPI channel along with the FSC threshold brought the negative population for DAPI back above 0 on the biexponential plot. These small DAPI-positive events, which were driving the negative signal, now surpass the threshold level in their own parameter rather than the FSC threshold.
|
Another solution is to decrease the FSC threshold low enough to make these events visible. However, this approach has its own challenges. It can be difficult to determine when the FSC threshold is low enough to capture these events, and it will significantly increase the number of unneeded events in your data, bloating the file size.
|
This issue is not common and required considerable intentional manipulation of both the machine and sample to demonstrate. However, it serves as an interesting example of the importance of the threshold parameter. It also provides valuable quality control information for future experiments. If your samples frequently have negative populations adjusting below 0 on the cytometer, even when compensation is excluded, it may indicate a staining issue that needs to be resolved.
|
|
For help adjusting or troubleshooting the threshold parameter in your next experiment, feel free to reach out to the FCF staff with any questions.
|
|
|
|
Publications & Seminars
- In Cytometry A, OMIP-105 got recently published and could be of interest to some of our users. This new optimized panel offers a 30 colors exploration of immune cell landscape in spleen and tumor within a syngeneic MC-38 murine colon carcinoma model. Feel free to check it out, it could save you time and money !
- Flowjo has an Upcoming webinar about High Dimensional analysis with Phenograph and UMAP on Thursday, August 15th (1 pm EST | 4 pm PST). If you are interested, you can register HERE.
|
|
|
|