|
|

|
|
|
Facility News
Hey all ! July is here and it brings rain and thunderstorms, what ? The weather might be a mess but the flow is always running smoothly at the FCF ! So please, enjoy our newsletter !
|
This month, as a follow up to the principles of compensation from June, we are talking about compensation beads ! Often misunderstood and ostracized from your experiments, we are here to put them back in your controls and explain to you why you should always consider them as an option. Even if they are a bit pricey, they can help you saving precious sample for the real staining !
|
the Flow Post-it ! We are explaining how to properly select the positive population in single controls. It will drastically improve your compensation with just a tiny slide !
|
Finally, I hope you are taking the opportunity to follow the Aurora remote training we offered last week. It is free and has multiple sessions. So please give it a chance and come see us for the training on the machine !
|
|
|
|
FACS Tips
Compensation Beads
With all the time and effort that goes into an experiment, it’s undesirable to lose precious cells to single stains, especially when it's for weakly expressed markers. This can create challenges in the compensation process that can have trickle down effects to your data. Compensation beads are an excellent alternative to using cells for single stains, and provide many advantages. Made up of two polystyrene bead populations, a negative control bead which does not bind antibodies, providing a negative peak, and a positive control bead that will bind almost any antibody non-specifically for a positive peak.
|
Pros
Compensation beads can quickly and efficiently be stained, saving you from using cells, and because antibody capture by the beads is very high, you can even reduce the amount of antibody added for staining controls while still producing a very bright signal. They also allow you to compensate with the same antibody being used in your experiment, which is not always true for antibodies of weakly expressed markers when staining cells. Also, the beads carry the same autofluorescence values, reducing the factor that it can play in compensation. It is also possible to use compensation beads for some controls and cells for others within the same experiment.
|
|
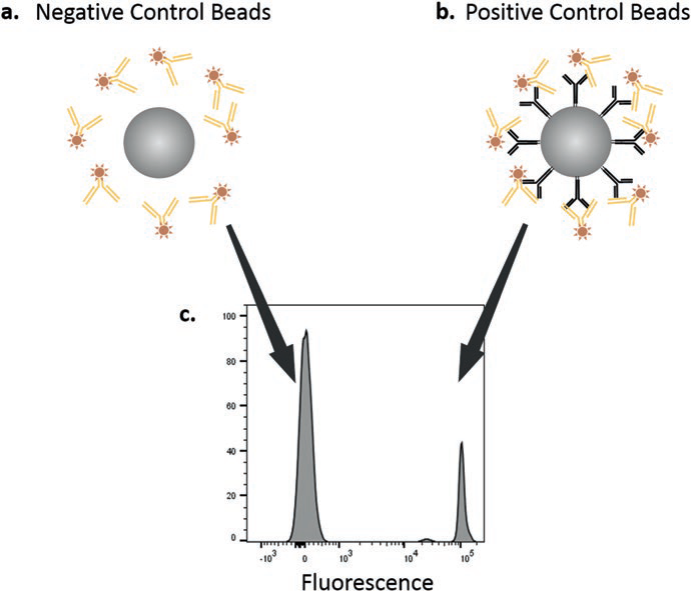
|
Goetz, C., Hammerbeck, C., & Bonnevier, J. (2018). Flow Cytometry Basics for the Non-Expert [electronic resource] (1st ed. 2018.). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-98071-3
|
|
|
Cons
Compensation beads do have shortcomings to be aware of. Compensation beads are not always reactive to antibodies raised in certain species, for example BD OneComp eBeads react to only mouse, rat, or hamster antibodies, so it’s important to check the manual of the beads you use. Additionally, some beads are only designed for a limited set of fluorophores and lasers, for example BD OneComp eBeads are not designed to work for the violet and ultraviolet laser, while BD UltraComp eBeads are. It is also the case that the emission spectra of fluorophores can be different between beads and cells creating variability. It is important to not base voltage values off compensation beads as they often do not reflect the same staining in cells. Oftentimes the fluorescence is much brighter in beads resulting in unnecessarily dimming the voltage which can result in worse population separation in your sample. Lastly, compensation beads will not work for viability dyes, so cells will need to be substituted, there are however special compensation beads designed for use with amine Live/Dead stains like LIVE/DEAD Fixable Aqua stain that can be used.
|
|
Conclusions
|
Compensation beads are a valuable tool in flow cytometry and can vastly improve the quality of your data. Just make sure they meet the needs of your particular experiment, different companies will provide specific bead sets that may better cater to your experiment.
|
|
|
|
Flow Post-its
Social medias are great source of information even when it comes to flow cytometry. A great resource is the twitter account of the Flow Cytometry Core Facility at Memorial Sloan Kettering Cancer Center (https://twitter.com/FlowMskcc). We thought it would be nice to highlight those tips and bring them to you !
|
|
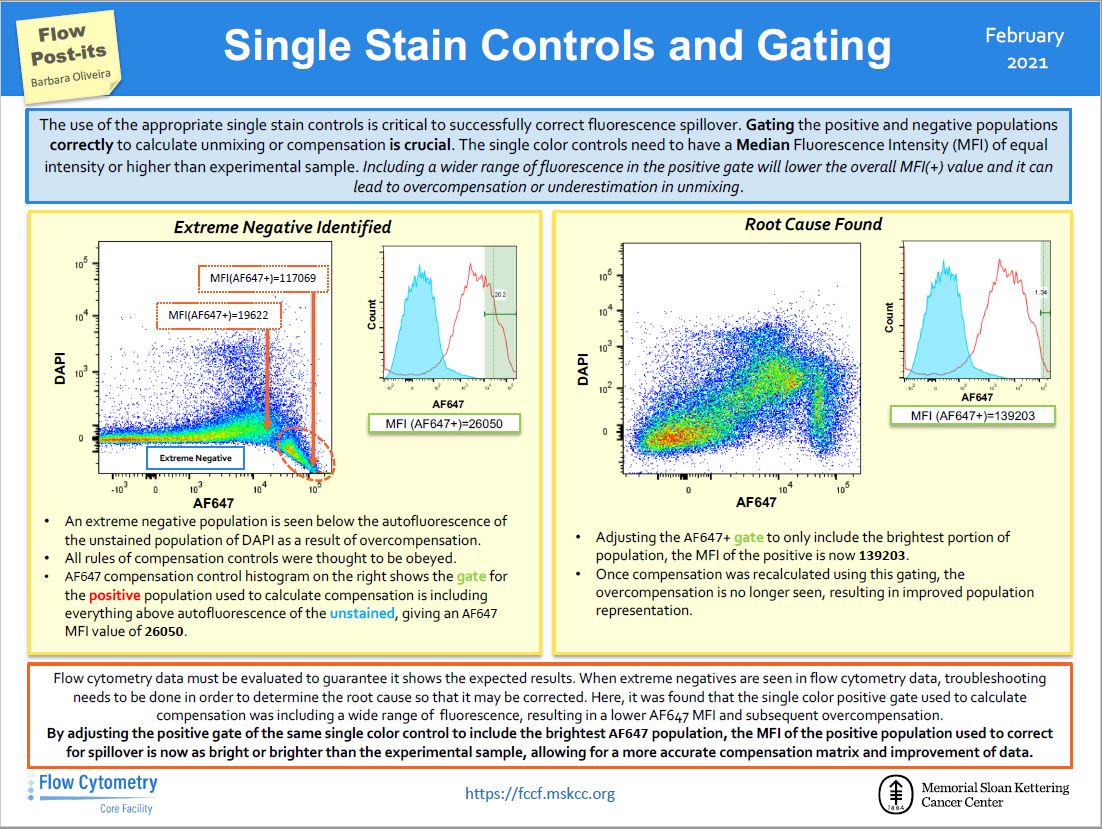
|

|
|
|
|
|
|