|
|

|
|
|
|
|
Facility News
|
It is finally that time of the year ! Remember when you were kid, waking up on Easter day and running in the backyard to pick up the chocolate eggs 🥚 and rabbits 🐰 and whatever form of chocolate was trendy that year (I heard flamingos are totally in this year). Well, don't you worry, I got you back ! Nobody said you had to stop being excited by chocolate hunts cause you are a grown up scientist ! I took on my duty to hide a decent amount of chocolate eggs in both facilities, so find them, let me know and most importantly SNACK them !
|
|
In this month FACS Tips, we are highlighting a useful approach to one of the most dreadful aspect of the perfect flow cytometrist : antibody titration ! I hope it will give you some ideas and willpower to do it !
|
|
Nuria Crua Asensio won the mug this month, Congratulations !
|
|

|
Each month, we will give away one of those special and unique mug designed by the FCF team. Answer correctly to our 3 questions and you can have a chance to win !
Please take few minutes to answer the quiz HERE.
|
|
|
|
FACS Tips
|
Combine to Save Time : Combinatorial Titrations
|
Often overlooked and underappreciated, titrations can be the missing piece to a successful experiment. The meticulous process of staining antibodies at varying concentrations and calculating the stain index to determine the optimal concentration is a vital step in experiment preparation. Titrations ensure the greatest separation between our positive and negative populations without oversaturating, potentially leading to cost savings by revealing that less antibody is needed than initially assumed. This becomes particularly evident when transitioning from smaller panel experiments to those with 20 colours or more on spectral machines.
|
|
But this is a double-edged sword, with more colours and complex panels comes the challenge of titrating a greater number of fluorochromes. For example, if you have 20 colours and do 6 concentration for each, that amounts to 120 tubes. It can be daunting to imagine having to titrate that many fluorochromes. It’s hard to blame ourselves in these situations for just rolling the dice with a best guess in antibody concentration and pushing on. Yet, in an increasingly spectral world, flooded with new fluorochromes designed to expand spectral panel capacities, a simpler solution is in demand. Addressing this challenge, Olivia Burn and Co. have authored a paper exploring the ability to perform multiple colour titrations within the same tube titled “Combinatorial antibody titrations for high-parameter flow cytometry”, which will be the focus of today's newsletter.
|
|
|
|
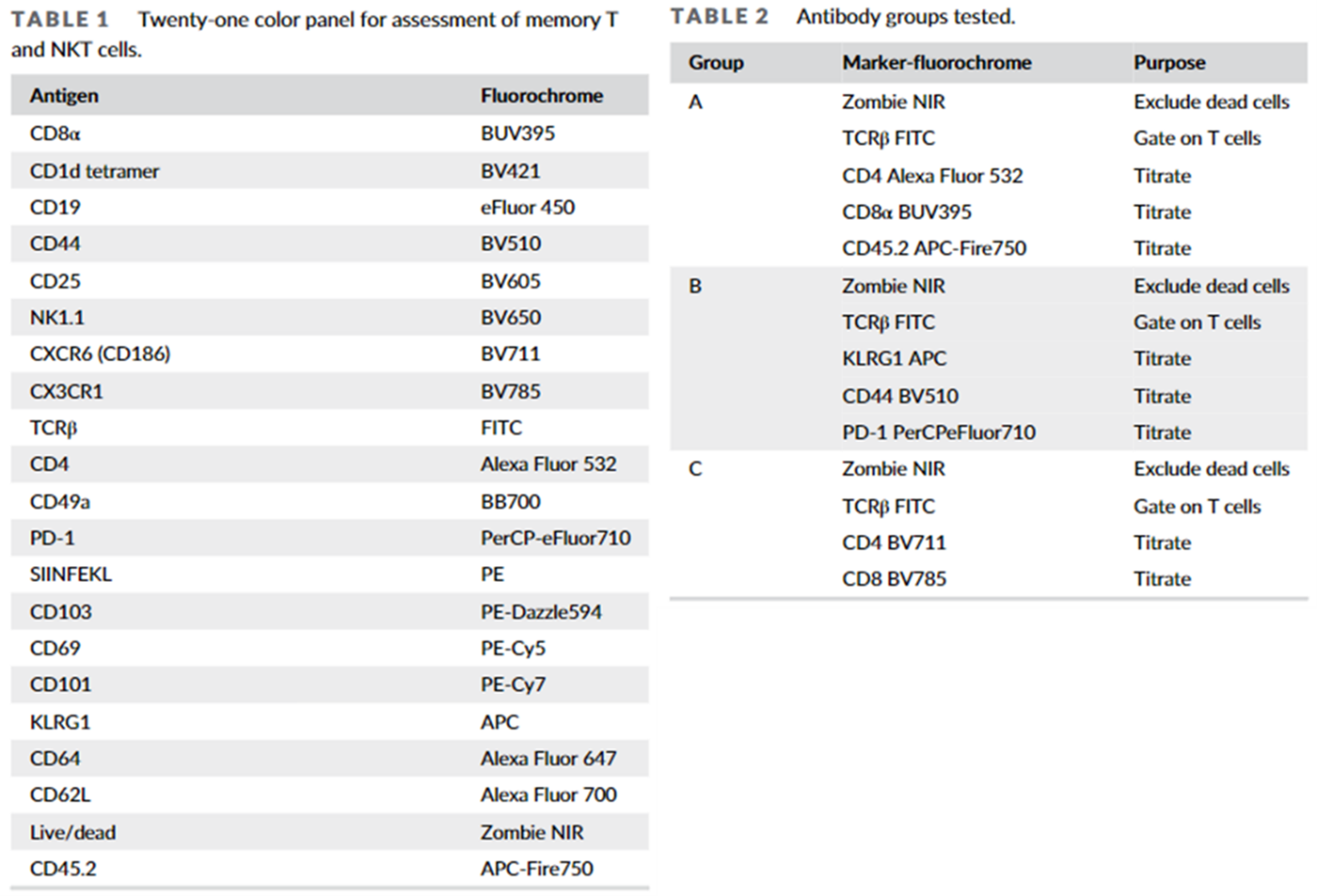
In their study, Burn et al. performed combinatorial titrations on a 21 colour T cell panel, grouping 3 to 5 antibodies simultaneously. They categorized these groups to demonstrate the influence of spillover spreading. Group A comprised highly expressed markers with little spillover, Group B contained low/mixed expression markers with some spillover, and Group C was intentionally designed to have a high degree of spillover between markers. Notably, each titration group also contained a viability marker and a lineage marker, these were included to improve sensitivity of the markers and better assess valid biological expression of the markers of interest. These 2 markers were also titrated separately before.
|
|
|
|
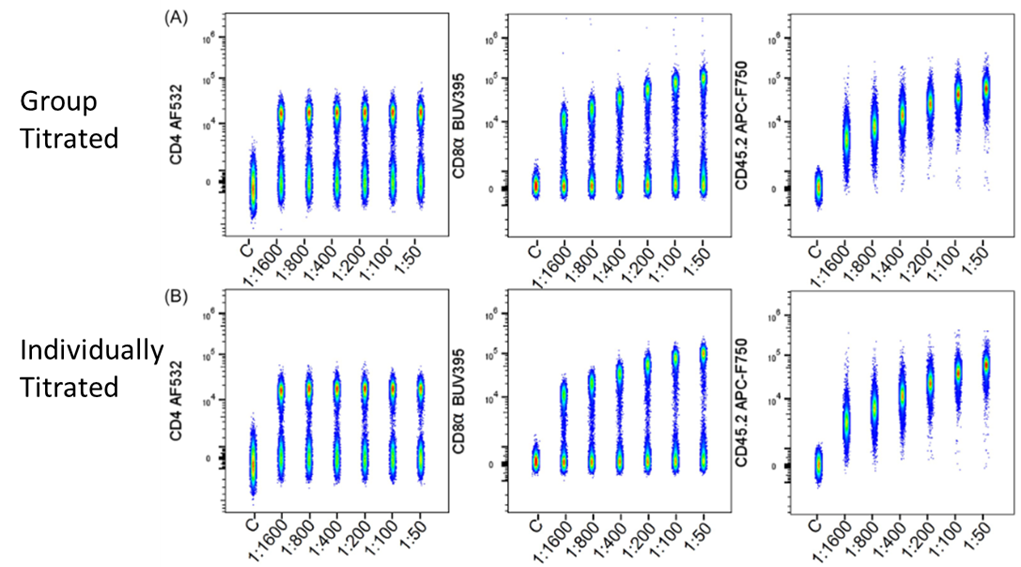
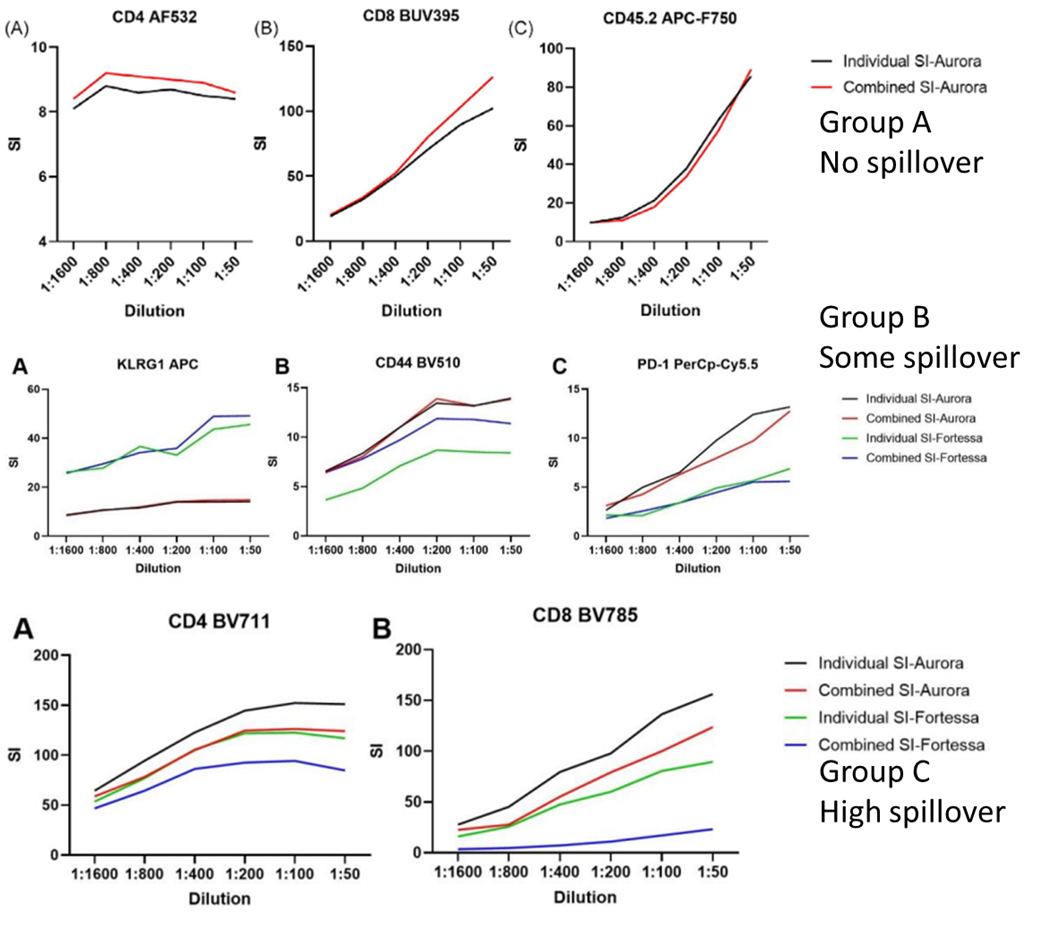
Comparing the stain index between titrations conducted as a group and individually, for both Groups A and B, they observed the same optimal saturating concentration. Even when done with a small spreading error between titrating markers, as was occurring in Group B, this held true. However, when highly expressed markers, with a high degree of spillover were combined in titration groups, they underperformed compared to their individually titrated counterparts. The spreading error's influence on the negative population in these groups has a heavy influence on the stain index calculation. The takeaway from this is that the same panel design principles that are essential for full panels, are also important to keep in mind here. For example, it is best to co-titrate antibodies with small amounts of overlap/spreading.
|
|
|
In conclusion, the authors provide three main guidelines for performing combinatorial titrations, which I’ve included below;
|
- During panel design, follow best practices to allocate markers to fluorochromes in a way that minimizes spectral overlap and spreading error.
- For grouping during titrations, primarily select antibody-fluorochrome conjugates that have low similarity indices and no or negligible spreading error based on the SSM.
- Group overlapping fluorophores with high spreading error only if assigned to markers that are mutually exclusive.
|
While the focus of this paper was for spectral machines, this process will also work for conventional machines, as they also included the Fortessa in some of their tests. For more information, definitely give this paper a read as there is much more in it than I can cover here. As always, feel free to reach out to the FCF staff if you have any questions.
|
|
Publications
This month our recommendation is the PAPER discussed today for the newsletter.
|
Burn OK, Mair F, Ferrer-Font L. Combinatorial antibody titrations for high-parameter flow cytometry. Cytometry A. 2024 Feb 6. doi: 10.1002/cyto.a.24828. Epub ahead of print. PMID: 38317641.
|
Presentations
|
April 15th Session 5 of the Swiss Lunch Cytometry Program presentation
|
Multi-omic characterization of different cell populations using the BD Rhapsody single-cell system. Tatiane Gorski, University Zürich
|
April 18th 10h GMT+1 FlowJo webinar: QC and Normalization
|
|
|
|
|
|
|