|
|

|
|
|
|
|
Facility News
It is the very much dreaded month of February ! Where Valentine's day ❤️could be trouble to your romantic life if you dare to forget it ! So instead of stressing you our about bringing chocolate, flowers or gifts to your loved one, why don't you take a minute to celebrate that unique relationship with your favorite cytometer ? I know each user have their very much their "machine", the one that never clogs, never run out of sheath and most importantly never gives bad data. So please, on that very special day, take a minute to send gratitude to your special one (and give love to the BD machines as we all know you will be picking the Auroras 😉).
|
|
Quick reminder : we would like to ask your opinion on how we are doing at the moment and what we could do to provide a better service. Please take a few minutes to fill up our 2024 feedback form ! You have until February 29th so you have the time to come up with suggestions.
|
|
In this month FACS Tips, Kevin is reviewing a paper that evaluates the potential limitation and problem arising from compensation beads usage in spectral cytometry. Have a good read and as always, we can help you anytime !
|
|
Eleonore Wolters won the mug this month, Congratulations !
|
|

|
Each month, we will give away one of those special and unique mug designed by the FCF team. Answer correctly to our 3 questions and you can have a chance to win !
Please take few minutes to answer the quiz HERE.
|
|
|
|
FACS Tips
|
Compensation Beads and Spectral Cytometry: A Potentially Problematic Pair
|
|
Compensation beads have become a reliable tool in our flow cytometry arsenal, a crutch upon which we can constantly rely. A quick and easy way to get bright positive populations, allowing us to overcome limitations in sample quantity or availability of a certain rarely expressed marker. However, this has increasingly proved to be problematic in spectral cytometry. For reasons not fully understood, compensation beads have underperformed expectations on spectral machines, creating inadequate unmixing matrices that produce suboptimal data. This is a phenomenon commonly witnessed on the Aurora. Even in situations where compensation beads you would think would be a necessity are avoided. For example, the much publicized 40 colour OMIP-69 was unmixed using single stains from cells only, this is also true with the 41 colour OMIP-93, and these panels are heavily optimized so we can be sure this decision was taken quite carefully. So, while there does appear to be a problem with compensation beads in spectral cytometry, which beads? There are plenty of vendor options to choose from. Then there are also differences in what markers are combined with the beads? All this can create much confusion.
|
|
|
|
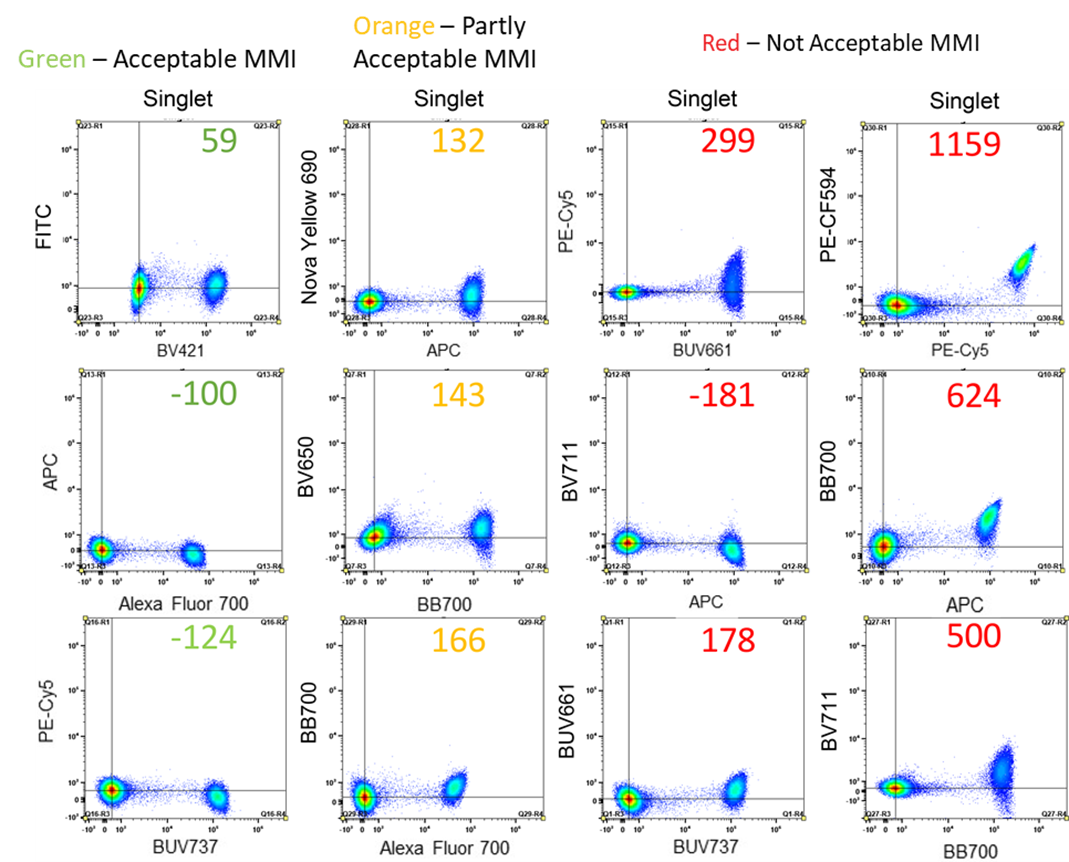
The American group of Debajit Bhowmick has taken on the challenge of solving this in their recently published paper “Side-by-Side Comparison of Compensation Beads Used in Polychromatic Flow Cytometry”. They performed a comprehensive comparison by unmixing with cells, but also by 8 different brands of compensation beads. While they make many evaluations across the paper, the most important calculation to determine the difference between cells and beads for quantifying unmixing mismatches is the Median Mismatch Index (MMI). The MMI is essentially a way of calculating the spread of a fluorophore, either positively, meaning into a secondary fluorophore, or negatively, away from a secondary fluorophore, to make a quantifiable evaluation of the quality of your unmixing matrix and single stains. Based on the value returned from this calculation they set ranges for acceptable (Green), partly acceptable (Orange), and not acceptable (Red) for the spread of each fluorophore used.
|
|
|
|
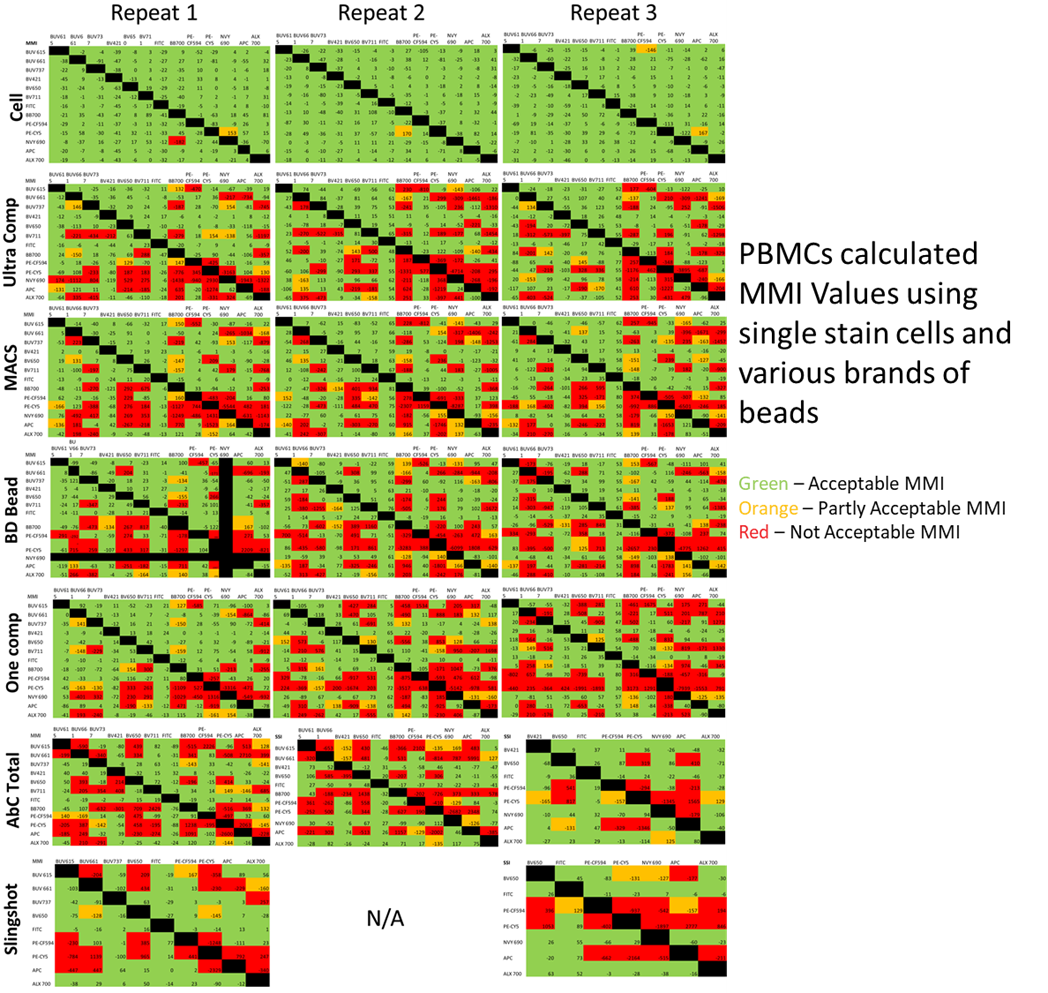
For their experiment they’ve used the Aurora as their spectral machine for comparison, and they intentionally picked fluorophores that you would expect a high amount of spillover to make the best determination of differences, but also included FITC and BV421 as a negative control for spillover. When they compared the unmixing of PBMCs for 13 fluorophores, in 3 replicates, for cells, as well as compensation beads, they produced the best unmixing by far when only using cells. While some beads outperformed others, some of the beads were excluded from this test as they didn’t meet the requirements for the rules of compensation to start with. All beads performed worse than cells, and the errors produced by the beads had a somewhat random quality to them. They occurred on both tandem and non-tandem dyes, and changed from one replicate to another. This makes the reasoning for why this is happening quite complicated.
|
|
|
|
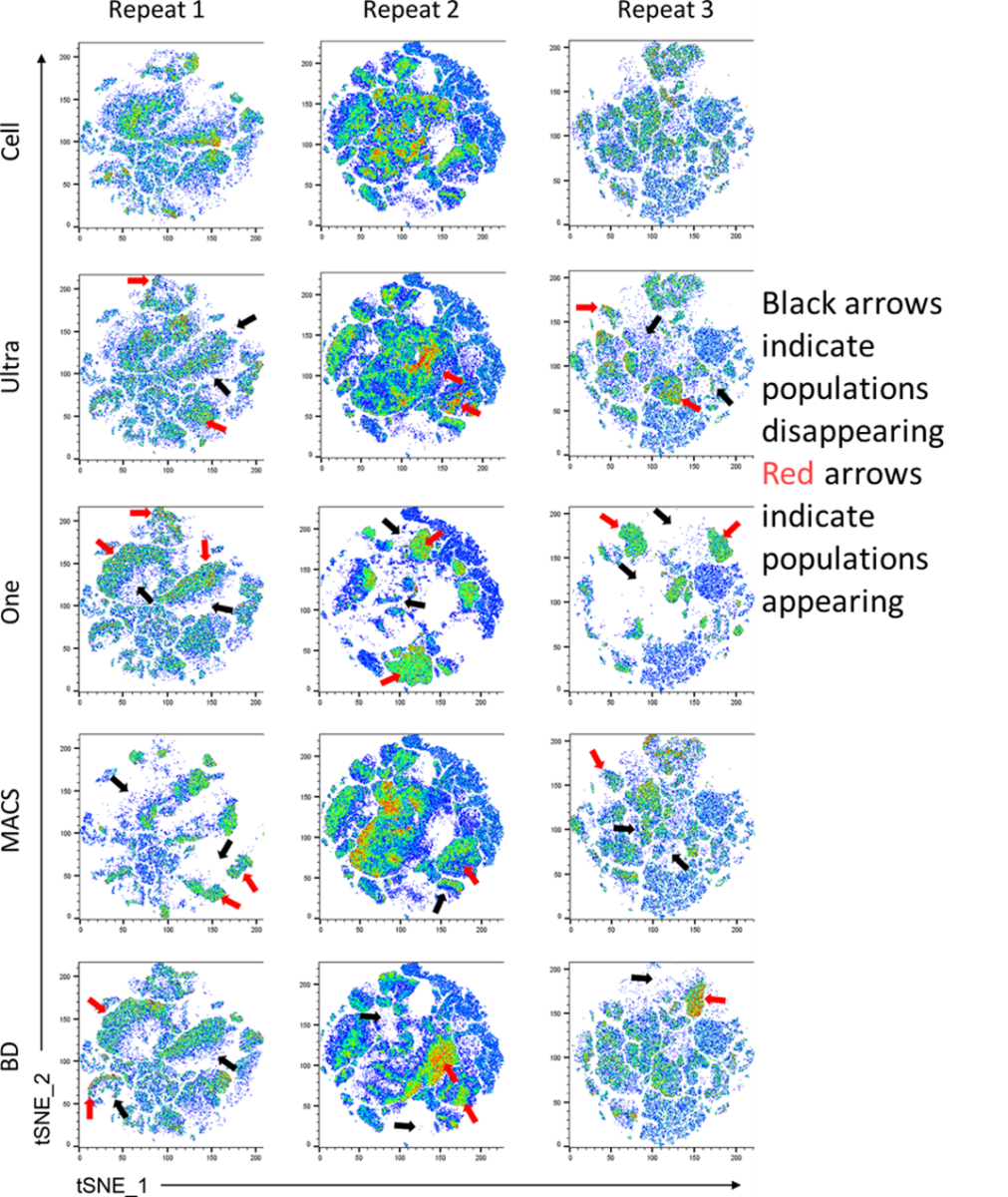
Using a simple 7 colour panel, the researcher looked at the similarity in tSNE plots produced with unmixing from cells vs various beads. Again, they noticed a number of population variance changes not only from cells to beads, but also between the different brands of beads. This means that two researchers with the same sample type, same panel, but different beads, could in theory get quite different results.
|
|
|
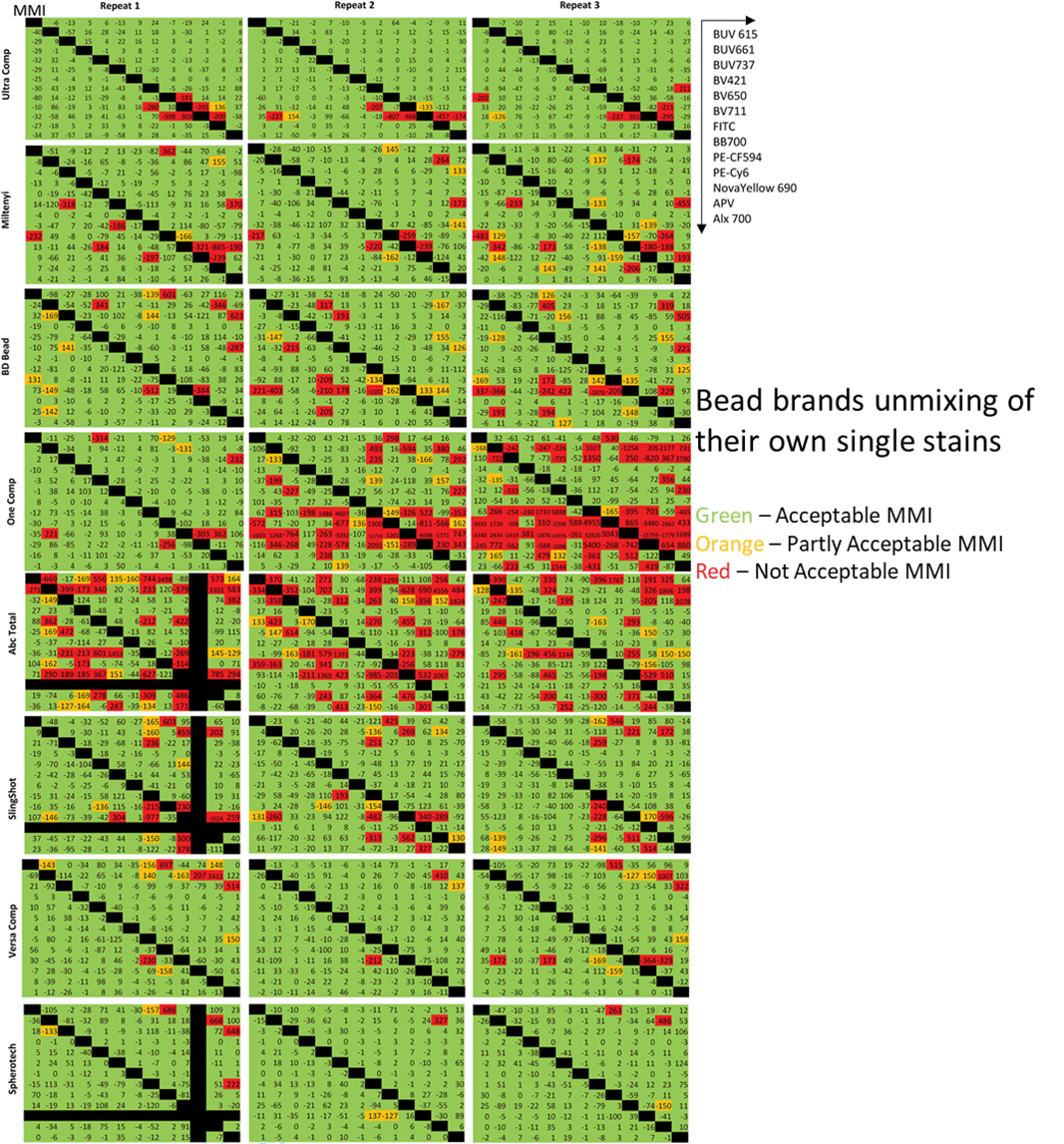
To determine a baseline level of variation in MMI, the researchers unmixed each bead lot’s single stains, with its own bead unmixing matrix, this would remove the influence of any inherent differences resulting purely between beads and cells. Although not nearly as much as the unmixing of cells, the beads have some issues correcting themselves as well, producing some “not acceptable” red squares. Particularly, the OneComp and AbcTotal beads struggled in this test. There is no explanation for why this error would occur, and it certainly was a surprise to the researchers.
|
So where does this leave us? We certainly wouldn’t recommend you throw your compensation beads in the garbage. Conventional machines, utilizing their simpler compensation calculations, don’t seem to produce the same problems when using compensation beads. And while they identified many fluorophores that had “not acceptable” MMI values, some fluorophores do still work well with certain beads, the problem is you won’t know this until it’s tested. The authors definition of what is “not acceptable” may also be much more stringent than what’s required for your experiment, as long as positive populations can be easily separated from negative spillover then small errors in unmixing matrices may not be an issue for your experiment. Even if there are spreading issues related to unmixing problems, an FMO can often ensure proper gating.
|
At this point our best advice for you when preparing for your next spectral experiment is to bring all your single stains in duplicate, cells and beads. Anticipate that cells may outperform beads in most places, but not all. Where cells can’t be used for single stains, make sure to test out the beads you’re using. It’s possible that another brand of beads may be better for the particular fluorophore in your panel. If you’re interested in learning more, definitely give this paper a read as they go into much more detail than I can here. As always, feel free to reach out to the FCF staff if you have any questions.
|
Starting this month, we will give some recommendation of papers and upcoming presentations that we think you might be interested in!
|
Publications
|
This month our recommendation is the paper we talked about here
|
|
Side-by-Side Comparison of Compensation Beads Used in Polychromatic Flow Cytometry
|
Presentations
|
The Royal Microscopical Society Flow Cytometry Data course is running again soon (online). 26 - 29 February 2024 (1400-17:00 each day)
|
|
|
|
|
|
|