|
|

|
|
|
|
|
Facility News
I hope you are not too afraid of changes cause there will be a lot of shuffling in the facility this month !
|
First and foremost, it is with a heavy heart that I have to announce the retirement of our dear LSRIIb. We both agreed that it was time for it to go. Having so many aging BD machines in our lab means we have to fix it more often and the LSRIIb kinda agreed to, literally, give its body to Science. So it will be gone but don't forget him when the LSRII SORP is kicking its new SIP or the LSRIIa shows off a new pressure sensor ! The LSRIIb is going to be off the IRIS booking system on November 3rd in the evening. Come pay your respect !
|
On a lighter note, The Aurora 2 will be installed on November 14th/15th and we hope for it to be useable on November 20th. We will keep you updated on the installation progress.
|
|
In this month FACS Tips, Kevin provide a quick overview of Imaging Flow Cytometry, where it comes from and where its heading. This little teaser will definitely put you in the mood to watch the more in-depth seminar he prepared with Francisco next monday, November 6th at 12:00 PM. It might give you idea about your future experiment !
|
|
Lilac Drori won the mug this month, Congratulations !
|
|

|
Each month, we will give away one of those special and unique mug designed by the FCF team. Answer correctly to our 3 questions and you can have a chance to win !
Please take few minutes to answer the quiz HERE.
|
|
|
|
|
|
FACS Tips
|
Imaging Flow Cytometry: An Overview
|
|
"Seeing is believing."
|
|
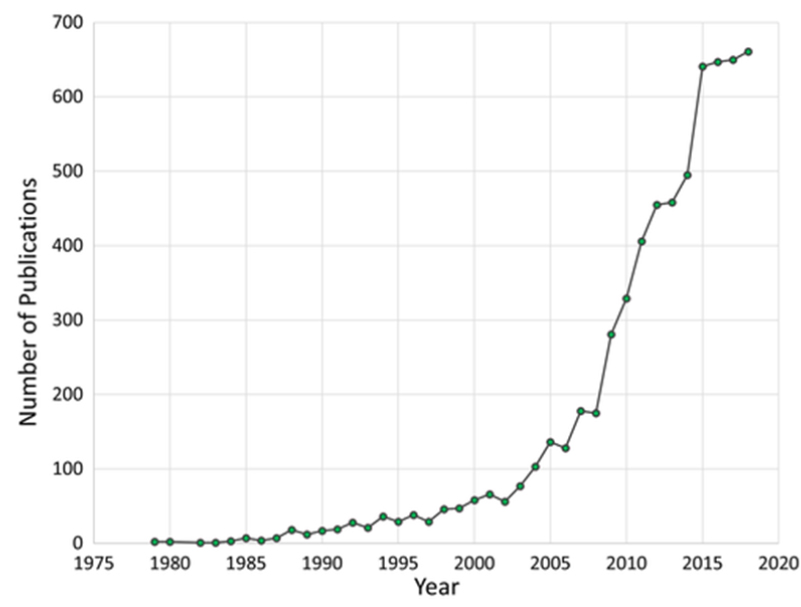
No presentation on the subject of Imaging Flow Cytometry is complete, or satisfactory, without this phrase. At least half jokingly according to Francisco and I. But, the jokes might be heading towards an end. The number of publications referencing Imaging Flow Cytometry has increased massively since the release of the first commercially developed ImageStream back in 2004, and more and more players seem to be entering the market. This makes ideal timing for Francisco and I’s presentation at the upcoming Swiss Lunch Cytometry Seminars on Imaging Flow Cytometry on November 6th. While this newsletter won’t be as in-depth, I’ll take the chance here to introduce our talk.
|
|
|
Image from Goda, K., Filby, A. and Nitta, N. (2019), In Flow Cytometry, Image Is Everything. Cytometry, 95: 475-477. https://doi.org/10.1002/cyto.a.23778
|
|
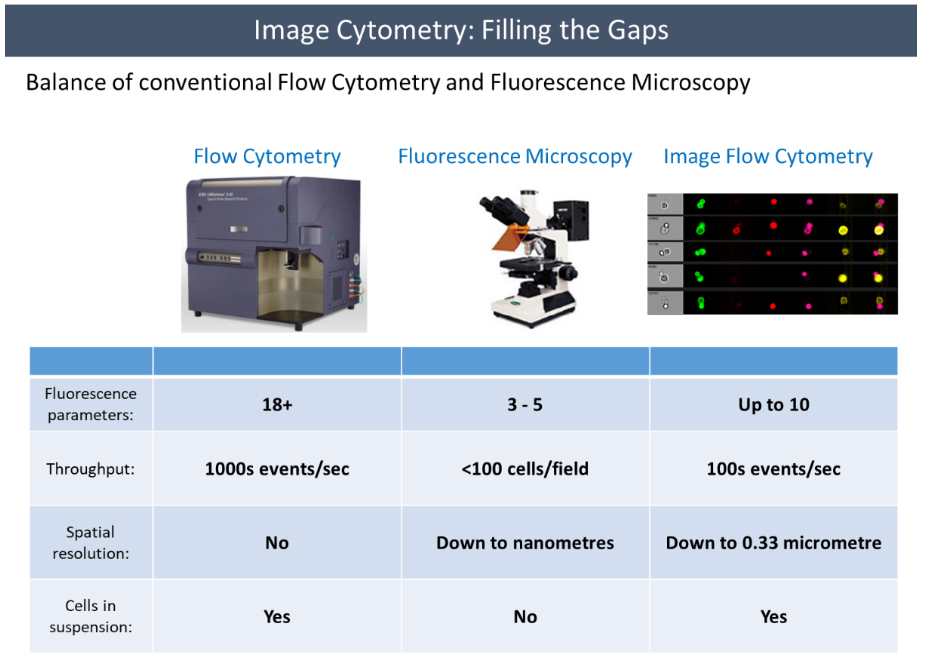
Flow cytometry is a widespread and powerful research tool. It delivers statistically robust results quickly, and now that modern machines can handle greater than 30 phenotypic and/or functional markers to answer our research questions, increasingly deep elaborate experiments are possible. However, it still leaves us at a loss in any experiment where spatial information is essential to our research question. Although techniques like FISH can be used, that is still an indirect approach to assessing location information. While we can move to fluorescence microscopy to achieve this information, we become limited, not just by the number of fluorophores we can use, but also in the ability to generate quantitative results. Microscopy can tend to be more laborious and subjective as well. Imaging flow cytometry fills this gap between microscopy and flow cytometry. It combines the speed and sensitivity of flow cytometry along with the spatial information of microscopy. This hybrid creates exciting new research opportunities.
|
|
|
|
It has to be said that at the moment there are limitations to imaging flow cytometry. For instance, while it is faster to analyze large populations of cells compared to conventional microscopy, it's still much slower than conventional flow cytometry, and it offers far fewer parameters. The fluidics of the ImageStream also make kinetic assays difficult. When it comes to image resolution, it again falls much shorter of what conventional fluorescence microscopy is capable of. So, experiments need to be designed carefully to make sure that these limitations won’t affect our experiments. However, with increased development in this field we may start to see these limitations shrink.
|
|
|
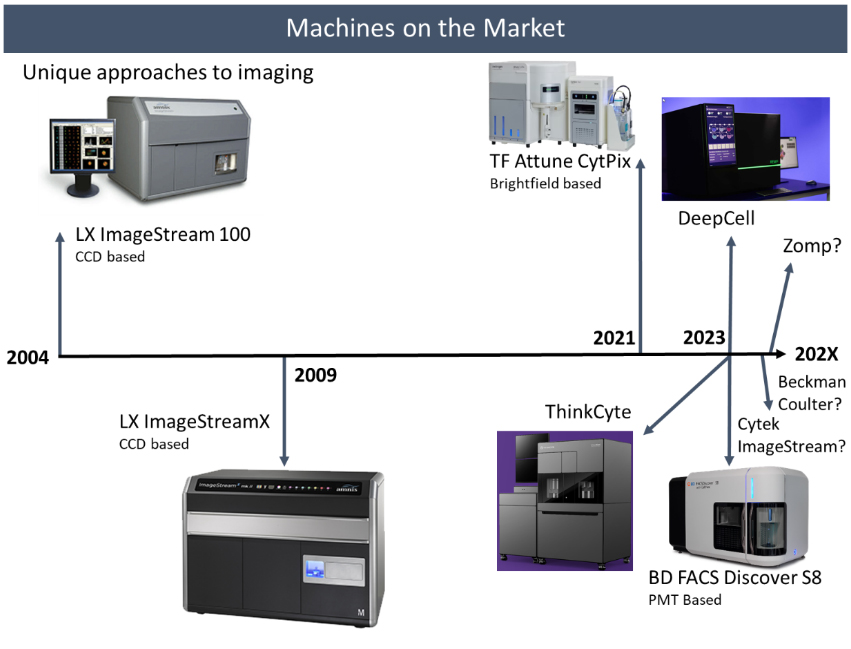
The first commercially available image flow cytometer, the ImageStream 100, was launched in 2004, with just 6 detectors and some design problems. It was followed by the ImageStreamX in 2009, which is the base version of the machine we have here at the FCF. This doubled the number of detectors to 12 and moved us up to 4 lasers. However, it was really only Luminex, now Cytek, that operated in the imaging flow cytometry space. This appears to be changing.
|
Thermo Fisher released a version of the Attune NXT cytometer in 2021 called the CytPix. This teamed their conventional high throughput flow cytometer with a high speed brightfield camera. While this is only a brightfield image, it did come with over 25 image feature parameters. We actually demoed this machine here at the FCF back in 2022, and the EPFL has one in its facility. While the imaging on this machine feels more of an afterthought, it has proved to be valuable for troubleshooting and quality checking samples at the machine. Becton Dickenson is releasing their FACS Discover S8 sorter this year, and since this was already the topic of the last newsletter, I won’t say much else other than it will also offer high speed fluorescence imaging, and offers the ability to sort off imaging derived parameters. So that’s two of the biggest companies in flow cytometry pivoting to imaging flow cytometry in the last few years. And with the acquisition of the ImageStream by Cytek, they too are looking to capitalize in this area. We have heard rumors that a new ImageStream will be available soon to try and keep pace with these challengers.
|
Then there are the various start-ups trying to get a foothold in the imaging flow cytometry space. At CYTO this year I was able to see two companies. First is DeepCell with their REM-I instrument (https://deepcell.com/product/), which offers high speed brightfield imaging and leverages their AI technology to perform cell sorting without labeling. Their brochure highlights 115 assessable dimensions of cell morphology, which sounds impressive. Next is the ThinkCyte with their VisionSort instrument (https://thinkcyte.com/product/). It relies on collecting the compressed spatial information required to produce an image, but due to the constraints of processing speed required for sorting it processes these measurements without producing the image using AI, such that sorting becomes possible. It looks at cells, not as a focused excitation point, but rather spread out across a meshed screen, which they call a structured illumination. This generates almost like fingerprints for the different categories of cells, which can then be selected and sorted. Another company we recently were introduced to has the very interesting name Zomp (https://www.zomp.one/). They appear to be in the beginning phases of producing an imaging flow cytometer capable of sorting.
|
|
Imaging cytometry extends the capabilities of conventional cytometry and opens up a window to exciting new research opportunities. And with an increasing number of both new and established brands trying to enter the field, it should make for some interesting advancements in this area. While it’s possible that these added features become just bells and whistle without much substance, it does seem clear that imaging technology is becoming a more standard feature in our conventional flow cytometry analysis. For a much more in-depth discussion of imaging flow cytometry including how the data is collected, analyzed, and possible applications make sure to join Francisco and I’s presentation for the Swiss Cytometry School on November 6th.
|
|
|
|
|