|
|

|
|
|
|
|
Facility News
|
It is finally THAT time of the year. That moment when your excitment can't be contained cause you finally got to the celebrate another year with your significant...flow cytometer ❤️. You know it, we know it. We all share a special bond with our LSRIIs or Auroras. Sometimes they have to leave us, a special thought for you, Gallios.That cytometer is unique, different and never breaks, according to your lab mates, and you've decided it will be the one for your time here. I am sure you always take good care of it but this month, take a moment to offer a blue reagent cleaning run to your machine and maybe remove your data from the hard drive to give it the lightness it needs for another year with you !
|
|
In this month FACS Tips, we are bringing to your attention an article that talks about overnight staining and how it could be of value to your research. Sometimes, it's good to take a step back and challenge established norms, especially if it helps you day flow better ;-)
|
There will be an online Cytek Spectral Happy Hour on Friday 10th of February 2023 from 2 to 4 pm. This event usually covers technical highlights and upcoming upgrades to the Aurora machine. If you are interested, please register HERE.
|
|
Marco Ongaro won last month quiz, congratulations !
|
|

|
|
Each month, we will give away one of those special and unique mug designed by the FCF team so please take few minutes to answer the quiz HERE.
|
|
|
|
|
|
FACS Tips
|
Increasing the incubation time, one approach to rule them all ?
|
For the amount of time we think about panel design, the simple act of staining is rarely more than an afterthought and seems fairly streamlined. Per tradition, 30 min incubation on ice in the dark is used for most surface staining. It is true that such procedure is often sufficient for most antigens especially those heavily expressed and targeted by high affinity antibodies. Yet, as we can attest by looking at flow cytometry data in publication, we can regularly see poorly discriminated signals that panel designs optimization can’t seems to improve on its own.
|
In THIS article published in Current Protocol, the author focused on the integration of overnight staining in the procedure and what would be its applications and restrictions to help improve the quality and consistency of experiments.
|
Increasing the incubation time for a better staining
|
Since we are talking about improving our staining resolution, it seems important to recall what happens when an antibody is binding its antigen. This interaction is a reversible reaction driven by non-covalent bonds. At first, the speed at which the antibody attaches to its antigen is greater than the rate it dissociates but when the reaction reaches its equilibrium, both rates become equal.
|
|
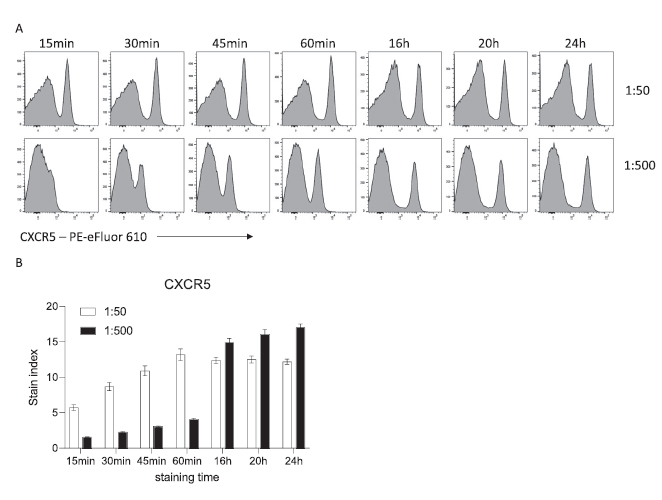
In conventional staining, cells are usually incubated with antibodies at high concentrations (µl/ml) for 15 to 60 minutes. Antibody binding follows a sigmoidal curve i.e. an S shape curve with an exponential phase leading to a plateau. The high concentration allows for substantial staining after only few minutes. However, it has been shown that the same binding level could be achieved with longer incubation times and lower antibody concentration. Indeed, when C57BL/6 mouse splenocytes are incubated with CXCR5-PE-eFluor 610 at 1:50, we can see a strong positive population after 15 min, reaching maximum MFI after 60 min. The same MFI can be obtained with 10 times less antibody over the course of a 16h overnight incubation (Figure 1).
|
|
|
Figure 1 Influence of time and antibody concentration on mouse CXCR5 staining. (A) Representative histograms of CXCR5 staining on viable C57BL/6 mouse splenocytes after the indicated incubation times. (B) Stain index (n = 5, mean ± SD).
|
|
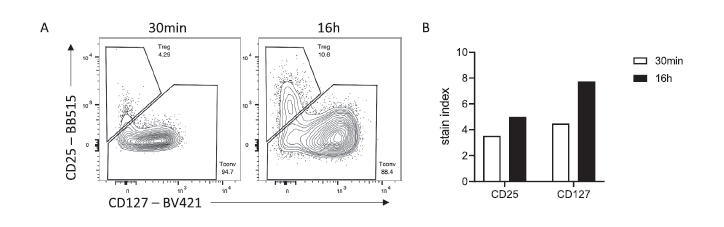
Longer incubation times can be of great interest for low affinity antibodies, hard to access targets (CD25, CD127) or antigens that gets internalized upon stimulation (CCR7) (Figure 2).
|
|
|
|
|
Figure 2 Superior discrimination of human Treg by overnight staining. (A) Representative flow staining of CD4+ CD3+ cells stained for 30 min or 16 hr. (B) Stain indices of CD25 and CD127 stained for 30 min or 16 hr. Data were acquired on a BD LSRFortessa cytometer.
|
|
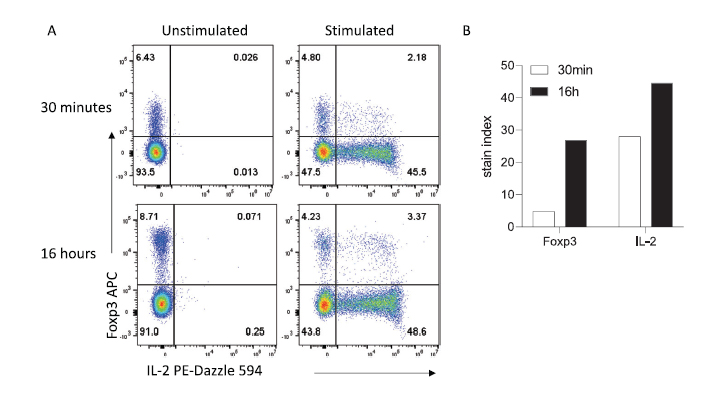
Furthermore, this approach works nicely for cytokines detection or intranuclear detection of transcriptional factors (Figure 3).
|
|
|
Figure 3 Intracellular and intranuclear staining can be improved with extended incubation times. (A) Representative staining obtained in 30 min versus 16 hr. (B) Stain indices for Foxp3 and IL-2. Mouse splenocytes were stimulated and stained for intracellular cytokines as described in the Methods section. Data were acquired on a Cytek Aurora Spectral Cytometer.
|
Finally, it is worth noting that using 10 fold less antibodies for a staining of similar or better quality can be a real benefit financially as some fluorochromes conjugates (BVs and BUVs) can be very expensive. Apply the reduction across your 10+ colors panel and this has the potential to drastically reduce the costs of your experiments.
|
Increasing the incubation time for a reduced interexperimental variability
|
|
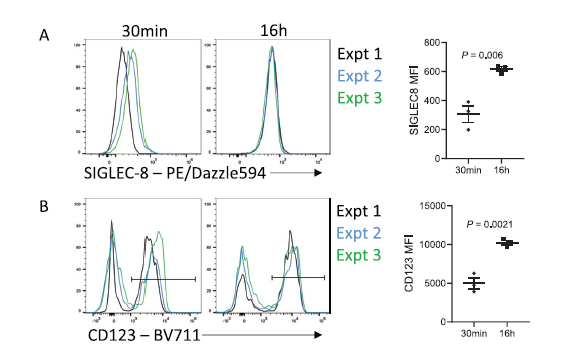
One of the not so obvious outcomes of extended antibody incubation times is the reduction of variability between independent experiments. As we mentioned earlier, antibody to antigen binding follows a sigmoidal curve. Since short incubation times (15-30 min) are often used in routine small differences in incubation times can have high impacts on MFIs variability. However, as we lengthen incubation times and step out of the exponential phase of the curve, we can expect much more reproducibility as we consistently reach the equilibrium of the on-rate/off-rate. (Figure 4).
|
|
|
Figure 4 Increasing incubation time reduces batch effects. (A) Representative staining and MFI of SIGLEC-8 on CD45+ SSChi CD16- cells from the same donor over 3 independent experiments. (B) Representative staining and MFI of CD123 on CD45+ SSClo CD3− CD19− CD14− CD16− cells from the same donor over 3 independent experiments.
|
If your experimental protocol is standardized thanks to a SOPs, the variability between experiments is negligeable and can even be of great interested for longitudinal studies with patient cohorts when it is pretty difficult to predict the sample arrival.
|
Increasing the incubation time without any issue ?
|
Although it is a very valuable techniques for the reasons we’ve just discussed, it is important to know that there are few caveats to mention. First of all, this new approach will require some time to perform the titration of each antibodies as the longer incubation do requires less antibody. It is also important to note that longer incubation might implies potential non-specific binding and therefore tests and setup might be time intensive at first. You can see in the article for more details about those issues.
|
|
Yet, the main issue that can rise when we talk about 16 hours incubation is cell viability. Although it is possible to fix the cells and not worry about it, many applications requires cells to be unfixed such as staining for chemokine receptors like CXCR5 or to prevent an increased background due to the fixation process in human samples. Luckily, for cells which are usually in good conditions as you prepare them, the incubation won’t have a dramatic impact on viability.
|
|
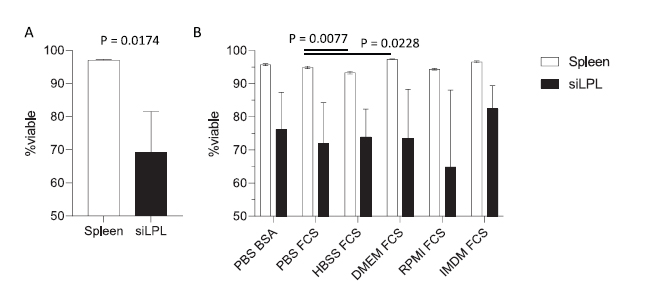
|
Figure 5 Buffer composition and preparation viability affects cell survival during overnight incubation. (A) Leukocyte (CD45+) viability prior to overnight incubation (n = 3, mean ± SD) in mouse spleen or small intestinal lamina propria leukocytes. Statistical analysis by unpaired t-test. (B) Impact of buffer choice on leukocyte viability in overnight incubation. Cellular viability was assessed on single CD45+ leukocytes that were negative for fixable viability dye prior to overnight staining.
|
As you can see, while splenocytes are not strongly affected by the staining time, small intestinal lamina propria leukocytes (siLPL) have a much stronger viability loss. Those effects can sometimes be mitigated though the use of an appropriate buffer (IMDM FCS vs RPMI FCS). Nonetheless, the viability changes observed being largely dependant on your cell type and staining conditions, it is imperative to test it out empirically in your model.
|
|
It’s exciting to see long standing conventions tested with new and different ideas as the authors have done in this paper and it definitely deserves a read. It might not applicable in every possible settings but this method shows that it can be interesting not only to improve your staining resolutions and experimental reproducibility but also allow for a more flexible daily workflow. As a final note, you will always be more likely to see a flow cytometer free in the morning at the FCF, so give it a try !
|
|
|
|
|