|
|

|
|
|
Facility News
Hey everyone ! We are back with a second issue of the FCF Newsletter. The first one was well received and we are very happy about it ! We will keep on bringing news about flow and tricks to improve your experiments !
|
This month, we've decided to provide some reminders about the Principles of Compensation. We are always happy to help people fix their issues but sometimes basic knowledge could easily save an experiment without our help. If you have any questions about it, please come talk to us !
|
The Flow Post-it ! is also related to the main topic as it illustrates the importance of correctly choosing your single stain dyes. You've been warned !
|
We would like to remind our users that we are always available to setup experiments and/or to run samples for them. It costs more than unassisted usage of the machine but it also gives more time to focus on your bench work. So next time you have to set up a 15 color panel, let us know and it will be done in no time !
|
Finally, if you have any subjects you would like for us to address in a future newsletter, feel free to send us an email about it !
|
Summer is around the corner and the quarantine restrictions are melting under the sun. Don't forget to enjoy some friendly time outside work as well !
See you next month 😉
|
|
|
|
FACS Tips
Principles of Compensation
Compensation can feel like an overwhelming process at times. More colours means more tubes needed, more attention needs to be paid to spillover, and larger spreading of populations between the positive and negative peaks. Then appears a dreaded overlap error and the process needs to be started over again from scratch, assuming there is still sample left in our compensation tubes. This process can be simplified though if we just focus on a few simple rules that streamline the process and get us up and running our experiments faster. Below are three simple, but not exclusive, rules to follow when performing compensation.
|
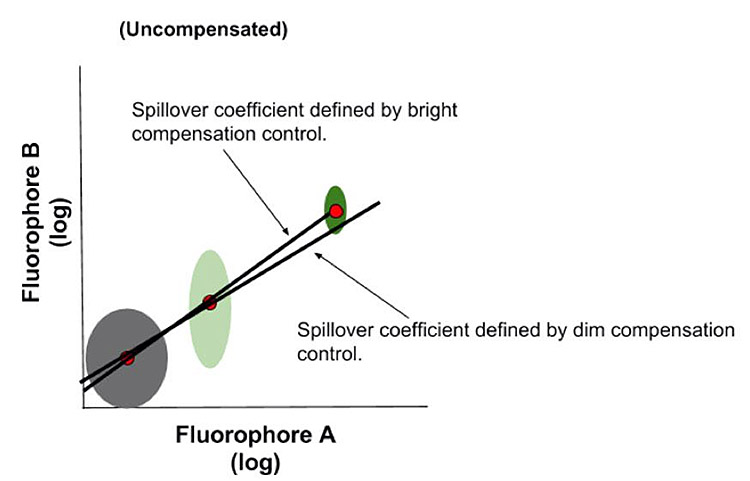
Compensation controls should be as bright or brighter (higher MFI for positive peak) than any sample the compensation will be applied to.
This is due to the fact that compensation values are calculated as a ratio of the difference of MFIs between a spillover channel and the primary fluorescent channel, and as the brightness increases in the primary channel there are fewer errors in this calculation. In practice, if we had a dim fluorophore single stain whose spillover values did not rise above background fluorescence, then little to no compensation value would be applied. If that same fluorophore in the sample was so much brighter that suddenly the spillover was above background, it would not be compensated for appropriately, and therefore would influence the data. Compensation beads can be very valuable for this purpose as they are often brighter than cells. Compensation beads will be a topic of a future newsletter.
|
|
|
Adapted from Bushnell, T. & Kissner, M. (2018). Advanced Topics in 4-10 Color Compensation for Flow Cytometry [electronic source] (1sr ed. 2018)
|
|
|
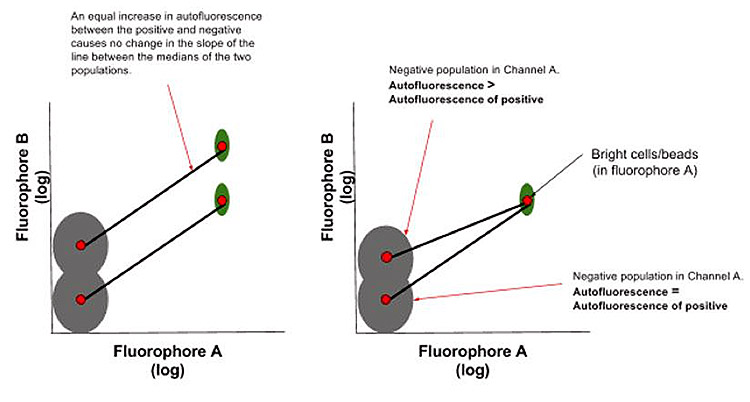
The autofluorescence should be the same between positive and negative peaks in each compensation control
Compensation is applied using a subtraction algorithm, therefore to negate an uneven effect it is essential that the positive and negative populations of a single stain have the same autofluorescence value, cancelling each other out and removing its influence on the creation of the compensation matrix. This rule still applies for samples without a negative population that require the use of a universal negative control. Again, compensation beads are valuable to comply with this rule as they have uniform autofluorescence in both positive and negative peaks.
|
|
|
Adapted from Bushnell, T. & Kissner, M. (2018). Advanced Topics in 4-10 Color Compensation for Flow Cytometry [electronic source] (1sr ed. 2018)
|
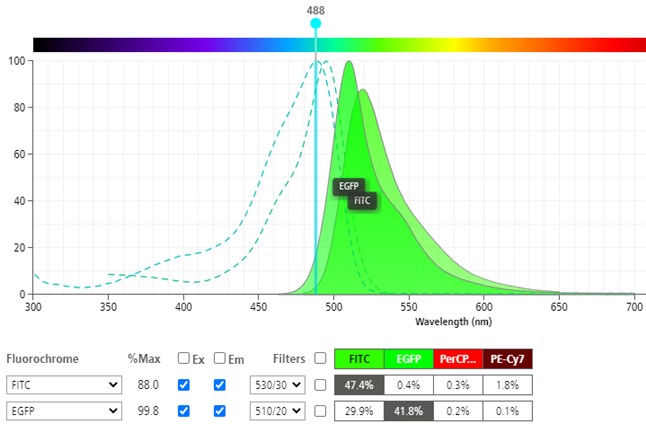
The fluorophore used in the experiment should match the fluorophore used in the compensation, also known as FITC ≠ GFP.
|
Different fluorophores have unique fluorescent profiles, meaning they spillover in different ways into surrounding channels. This is why spectral cytometry allows us to use certain fluorophores together that couldn’t be used before (Ask us about the Cytek Aurora). For this reason, compensation applied with one fluorophore will not calculate compensation correctly for another, even if they are similar. This is especially true for tandem dyes where variation of fluorescence spectra can change by lot number as well as over time due to vulnerability and degradation in the construction of the tandem.
|
|
|
Conclusions
|
These fundamental rules of compensation, when followed correctly, give us the best opportunity to get great data, however they are not the only rules. It is important that each fluorophore be strongest in its own channel, and compensation controls should be prepared exactly the same way as samples. Never hesitate to reach out to the staff for assistance setting up and performing compensation for your experiment.
|
|
|
|
Flow Post-its
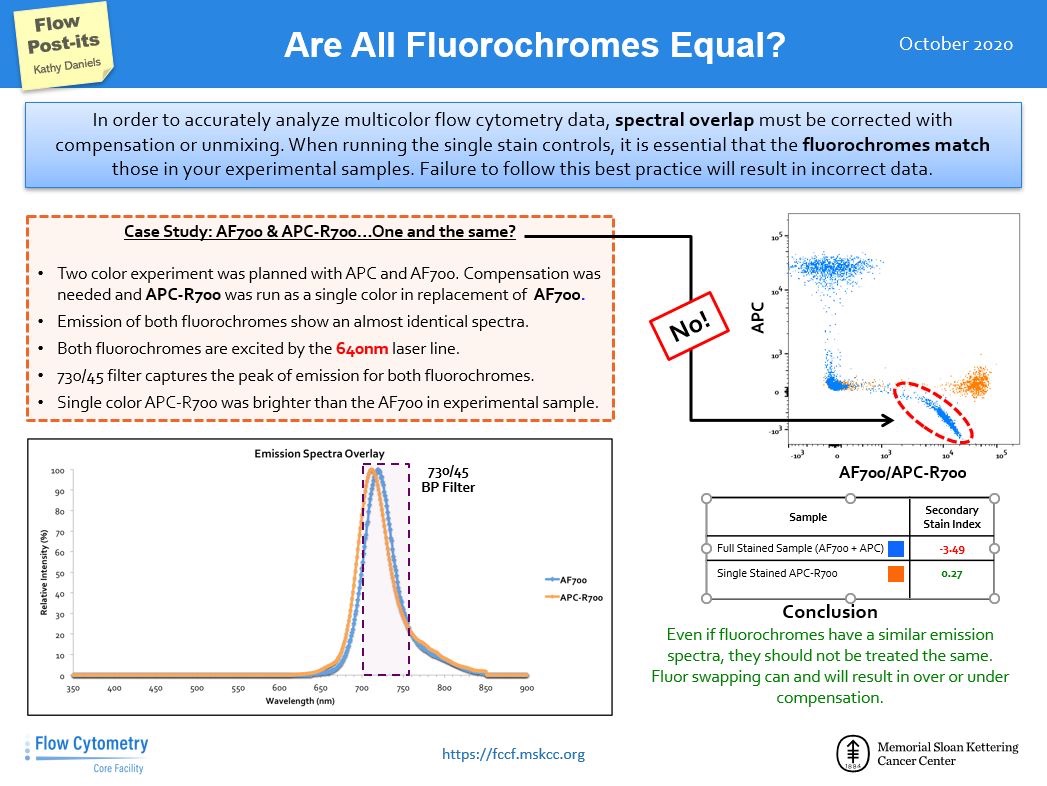
Social media is a great source of information even when it comes to flow cytometry. One useful resource is the twitter account of the Flow Cytometry Core Facility at Memorial Sloan Kettering Cancer Center (https://twitter.com/FlowMskcc). We thought it would be nice to highlight their tips and bring them to you !
|
|
|

|
|
|
|
|
|