|
|

|
|
|
|
|
Facility News
May the flow be with you cause it is for sure with us ! Yes, we are now at the Newsletter's 3rd year✨! Hard to believe there was a world without it, huh ? This year, we will continue bringing you new tools, new machine showcases and even topics you want to get covered ! Please, contact us if you have a subject you want to discuss here !
|
We took the time to analyse the results of the satisfaction survey we asked you to fill in last february. Thanks a lot to the users who took the time to reply, it is truly appreciated.
|
Overall, the facility has a good satisfaction level (86,9% max score) with an emphasis on the sorting being our top rating (90,7%) and the analysers earning a lower score (59,3%). This score is often related to how busy the machines can be and the dirty state in which some are left after a full day of running. We are going to be more vigilant about it and incorporate long cleanings when the machines are free to allievate this issue. Some recurring critics mentioned the fact the Epalinges analysers room is often cold 🥶 and we apologize about it but not much can be done about this part. It is mostly automated...Maybe we could bring lovely blanket for our users ?
|
|
Finally, a picture is worth a thousand words so imagine what a picture of a thousand words can be worth ? I compiled the words you associated with the FCF Facility and you can see the result below :
|
|

|
|
It is a relief to know that the efforts we put in to manage the facility are appreciated and valued by your guys, thanks a lot !
|
|
In this month FACS Tips, we are covering EasyPanel, an automated panel building tool that could save a lot of time for you and let you focus on other aspect of your research!
|
|
Mélanie Tichet won last month quiz, congratulations to you !
|
|

|
|
Each month, we will give away one of those special and unique mug designed by the FCF team so please take few minutes to answer the quiz HERE.
|
|
|
|
|
|
FACS Tips
|
EasyPanel
|
Panel design can be one of the most challenging aspects of flow cytometry. Although the idea is simple, we have to make best-guesses and anticipate how fluorophores, some we’ve maybe never used before, will interact with one another as far as spreading and spillover, compensation, brightness, etc. There are a variety of rules to keep in mind, and with every choice of fluorophore, there can be a downstream effect on another fluorophore that requires us to circle back and evaluate it all again. Get it right and we have an organized logical set of populations and cells set out on straight lines and crisp shapes. Get it wrong and we have weirdly streaking populations often pushed below zero into the axis, or spreading and overlapping another population. While we all like to think we have the time and place to carefully test and trial panels until we have it perfect, we often have to make do with what works to a reasonable degree right away. Thankfully, the group at EasyPanel has developed a tool to help tackle this challenge.
|
Their automated panel building tool algorithmically takes your markers of interest and the configuration of your machine and builds the most optimal panel possible. This web-based platform will try hundreds of thousands of different panel possibilities, considering complexity, similarity, and spillover for the all the available fluorophores and suggest for you the most optimized panel right away. Even if you have a pre-existing panel, and you just want to add 2 more colours, it is flexible to find the best way to work new fluorophores in.
|
Using the tool
|
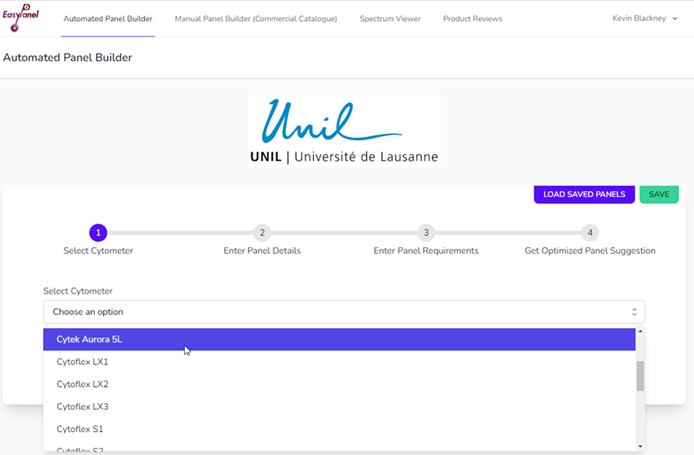
You can get to EasyPanel either linked from our website or by going to https://flow-cytometry.net/. Create an account with your @unil.ch or @chuv.ch email address. Once open, you should see the UNIL logo at the top.
|
|
|
First, select the instrument that you intend to use. We have uploaded the configuration for all our machines, and they should be available in the drop-down menu. The algorithm will perform slightly differently, and also give different options when performed for a spectral machine (Aurora) vs a conventional machine. For the moment you can use the preselected first option for the “Fluorochrome Data Source” question, however we will add our own spillover-spreading matrixes for available machines over time.
|
|
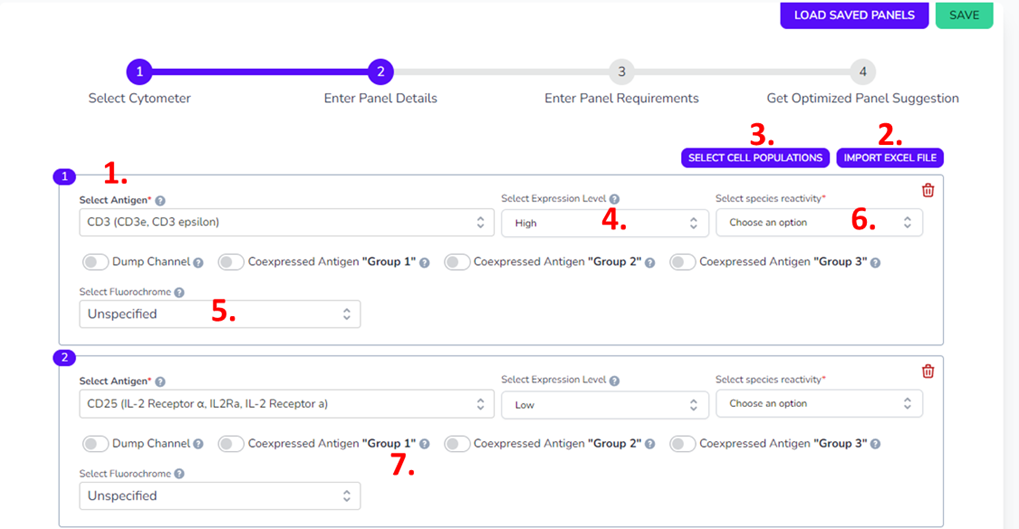
Next select the markers of interest. There are three different ways to input antigens, by manually typing them in (1.), import using an excel file (2.), or using the Select Cell Populations (3.) pre-set marker panels in the software available at the top, which will then give you a drop-down menu of choices. This Selected Cell population option is great for designing panels around the suggested markers of the latest publications in this area.
|
|
|
Some markers have already been updated to inform you on the degree of expression. For example, CD3 is listed as High (4.) while CD25 is listed as Low, however others are listed as unspecified. If you know the expression level of your specific antigen feel free to add this, or change it to unspecified if you think your antigen may be expressed differently than expected in the literature. At this step, you can assign a fluorophore to certain markers if they’re already known for some, for example if you have GFP or a predetermined tetramer (5.). It’s necessary that you specify the species you’re working with in the top right corner (6.).
|
While it’s optional to do, it’s useful to designate certain antigens as coexpressed, as this will better inform the algorithm to avoid putting coexpressed markers on fluorophores with greater degrees of spillover (7.). Coexpression is helpful in guiding the tool, it will tell the algorithm to avoid spillover more in these fluorochromes, however the software will already try and optimize this so it’s not completely necessary. You can designate up to 3 groups.
|
|
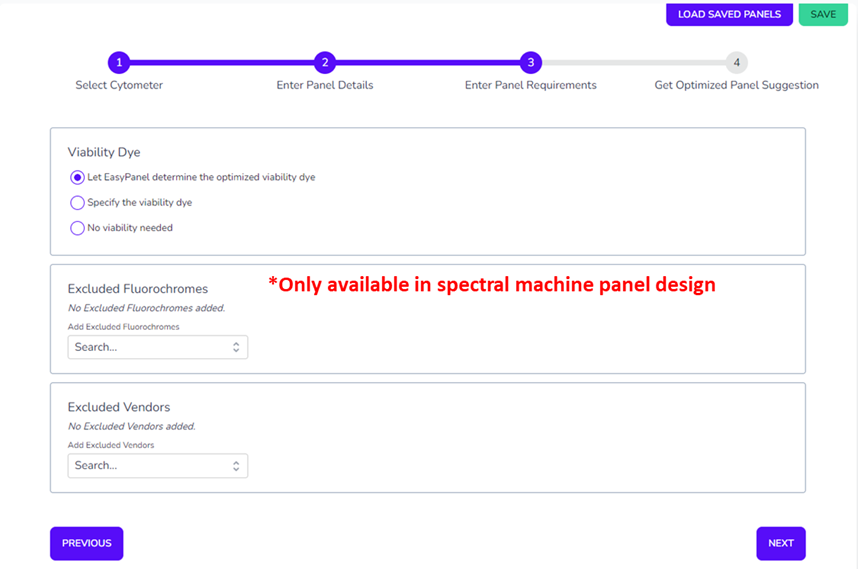
Avoid entering a viability dye at this stage as it will be asked for at the next step. You can either let the software select your viability dye, unless you know which one you will use already and input it now. You can tell the software to avoid certain vendors or fluorochromes if there are specific ones you’d like to avoid, either because they’re expensive or hard to source. The algorithm will work around forced or fixed fluorochrome pairs that you enter into the panel builder. Once you have submitted your markers and provided all the information, the software will return for you its calculated optimized panel.
|
|
|
|
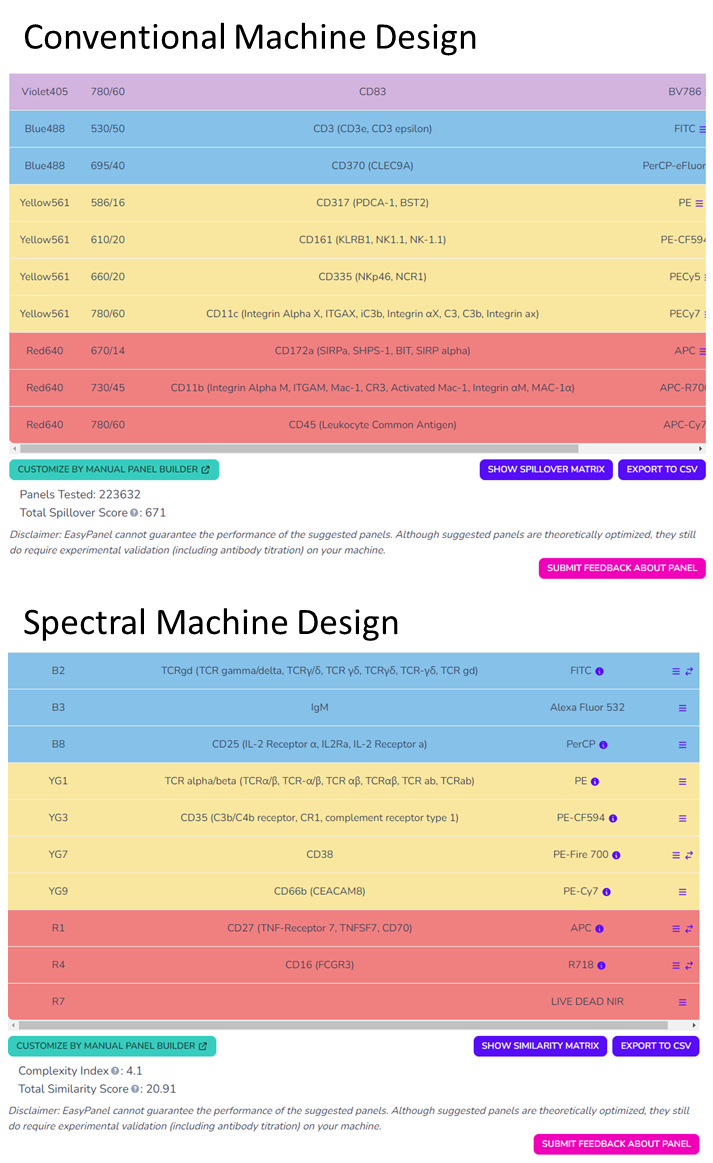
After a short processing time the EasyPanel algorithm returns an optimally calculated set of fluorochromes, displaying the determined Total Spillover Score (Conventional machine panel design) or Complexity Score and Similarity Score (Spectral Panel Design). You will also get a visual representation of the spillover matrix of the panel in a built-in spectral viewer, which is a nice way to check some of the spillover beforehand. Currently EasyPanel also pulls from a database of around 500 papers, and if your input panel shares 10 antigens or more with anything in the database it will also suggest a gating strategy for you to use, and list the relevant publications on the bottom of the page. So, while you may not be guaranteed a gating strategy now, this thoughtful feature may expand in the future to smaller panels.
|
|
|
|
|
By clicking on the tab in the Vendor column of each suggested fluorophore you find the expansive commercial product database where it will suggest antibodies for purchase from BD, Biolegend, ThermoFisher Scientific, Miltenyi Biotec, Beckman Coulter, Biotechne and many others. There’s a review option available as well to read other peoples reviews of the various antibodies, or if you would like to add your own. Finally, if there are any custom changes we would like to make we can move to the manual panel builder mode for this.
|
|
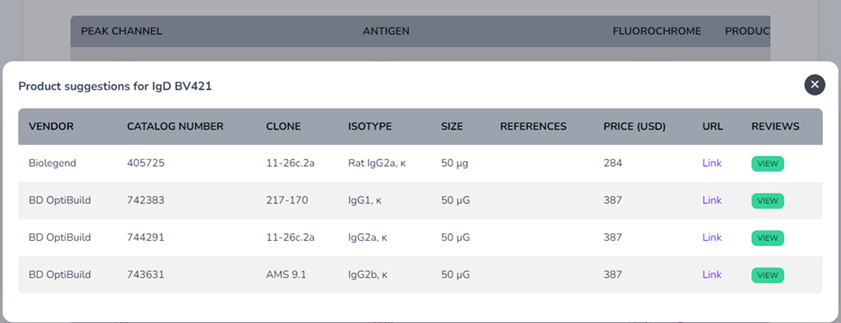
|
At the moment, one of the main complaints we have heard from people who have tested EasyPanel is that it doesn’t specify to the antibodies available in your pre-existing antibody inventory. We’re working on loading this as a selected Vendor category, so you can have panels designed only from this group in the future. Another issue is the algorithm often suggests more expensive new fluorophores over more classic ones. This is being addressed, for one, very large panels you may not have an option but to try newer dyes. For smaller panels, they are looking to add an option based on price, while also adding a new tab called inventory, where you can custom limit the options for antibodies based on your inventory currently available.
|
|
The team at EasyPanel has developed has developed a powerful tool for panel design, that should ideally get you to better data quicker. You can go ahead and create a free account with EasyPanel by @unil.ch or @chuv.ch email and test it out. We also have a webinar recorded that you can watch back for a more thorough guide (HERE). Please feel free to reach out to us for support, or if you’d like to get in contact with the EasyPanel team directly they would be happy to answer your questions.
|
|
|
|
|
|
|