|
|

|
|
|
|
|
Facility News
Oh yeah, September is here and I am fairly certain it brings back a lot of first day of school memories where waking up was nearly impossible after so many weeks of holidays ! Aren't you glad you don't get to have vacation and can keep enjoying the thrill of Science all year round ?🙃
|
In this month's FACS Tips, we provide some guidance to help you perfect your gating strategies and think outside the box to get the data you are looking for. Enjoy the read and feel free to ask us questions !
|
|
We are happy to announce a new BD machine is available at the Agora. The LSRIIc is located in the P1 Lab and has the same configuration as the Fortessa 2. It has been put together by Danny, flow cytometrist extraordinaire, Thank you !
|
|

|
We are bringing back the QUIZ ! As usual, the questions relates to the content of the newsletter. We made it even easier for you to fill up the form online as there will be a QR code next to each machine, just scan it ! While you are being an amazing user cleaning the machine, why don't you try to earn our monthly gifts ? In addition to the special Toblerone bar, we will add a personalised mug with a design from the team ;-)
|
Lastly, we are opening our Twitter account to make sure you can always get the most recent development in flow cytometry, so please follow us 😊 !
|
|
|
|
FACS Tips
|
Gating
At the end of the day, having to gate our cells can be one of the most frustrating parts of an experiment, samples look different from one to the next and there are irregularities that we can’t always control for. While some steps in the gating process will be standard no matter what the experiment, others require individual attention and can’t be applied uniformly. This creates the subjectivity that can cloud the process of gating, but can be alleviated if we take our time and have a consistent strategy.
|
|
You are in control of how you gate cells, and while there is not necessarily one correct way to analyze your samples, there are certainly wrong ways. Simplify the process down and work through your samples by checking quality, removing doublets and dead cells, and using control-based gating to determine your cells of interest.
|
|
Doublets
|
It’s a standard part of every analysis to remove any doublet events. No matter how good our preparation is, there will always be some cells that stick together, giving us false positive events, or unnatural populations. There are several ways to remove doublets using the FSC and SSC parameters, oftentimes it’s useful to combine a few of them as shown below.
|
|
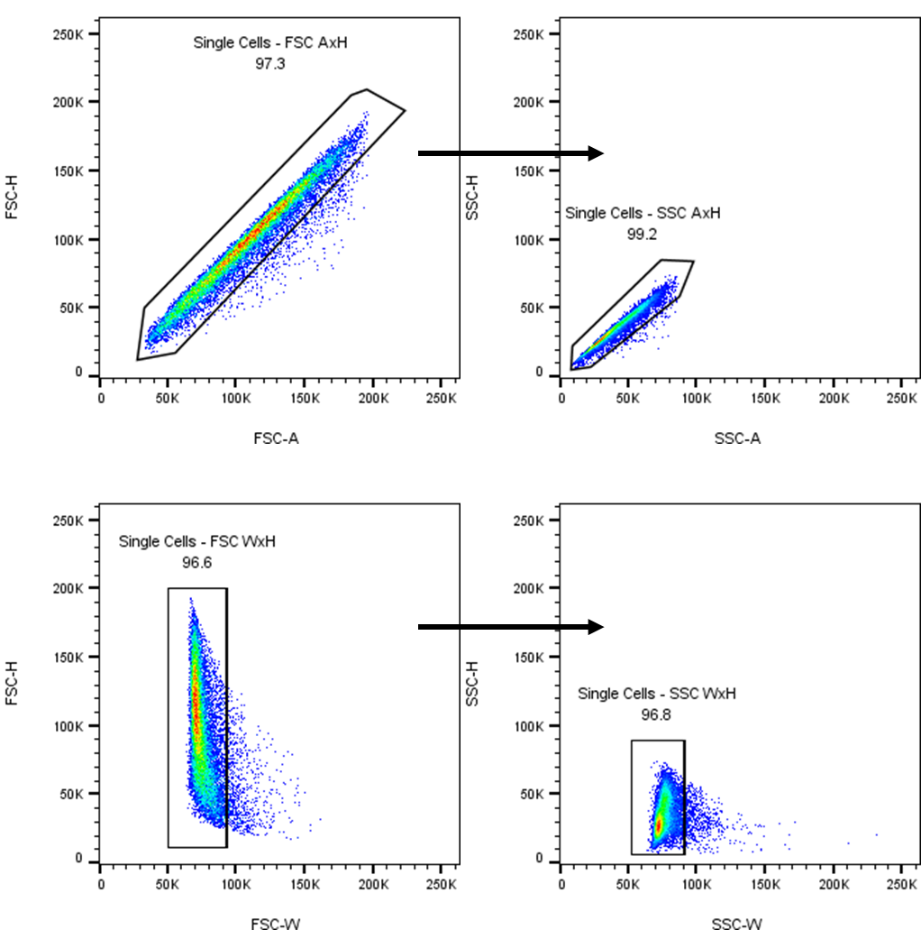
|
Adapted from Union Biometrica
|
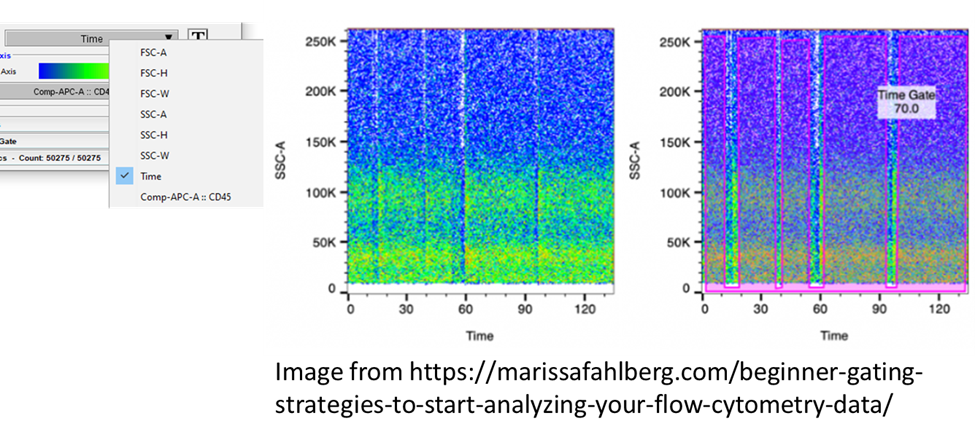
Time
|
It’s a common occurrence, we start recording a tube, we get distracted and before we realize, the machine has been running an empty tube for awhile. While this doesn’t seem like a big deal, when it comes time to analyze this file, the introduction of air into the machine, or any kind of fluidic instability can mess with our plots, give us false positives, and lower the quality of our experiment. Air can scatter and reflect light in the machine giving the effect of randomly scattering cells and fluorescence, but we can get rid of this. An often overlooked part of preparing a data analysis strategy is using the time gate. Time is always collected as a parameter on the cytometers. If we know that a small clog or air has been introduced into the sample, and we want to remove it from our analysis, this can be done by creating a time plot to identify and gate out these elements which will appear as unusual spikes or gaps in the data. FlowJo has even added plugins that will do some of this data cleaning for us. Both FlowClean and FlowAI plugins can be run on your samples before analysis and will remove abnormalities in your data so you can get straight to analyzing your samples.
|
|
|
Dead Cell Exclusion
Including a viability dye is an incredibly valuable part of any flow experiment, and should be included within the first couple gates of any analysis strategy. Because dead cells can bind non-specifically to your markers, it can give you inaccurate populations so they need to be removed before looking at other markers. For more information about viability dyes, check out our previous newsletter on the topic.
|
Loose Gating and Backgating
Sometimes, when we get our prep just right, we have an excellent FSCxSSC plot that clearly separates cell populations right away. Since this isn’t always the case though it can be better to start with a loose gating approach, including more than our population of interest. This can be helpful in multiple ways, first being that we won’t potentially miss our cells of interest by starting too narrow. Also, if we include different cell types that don’t express a marker of interest they can provide a more obvious negative population to guide our gating further along in the process. Once we’re happy with our gated final population it is always possible to backgate through our analysis and tighten gates and make adjustments if we see anything out of place.
|
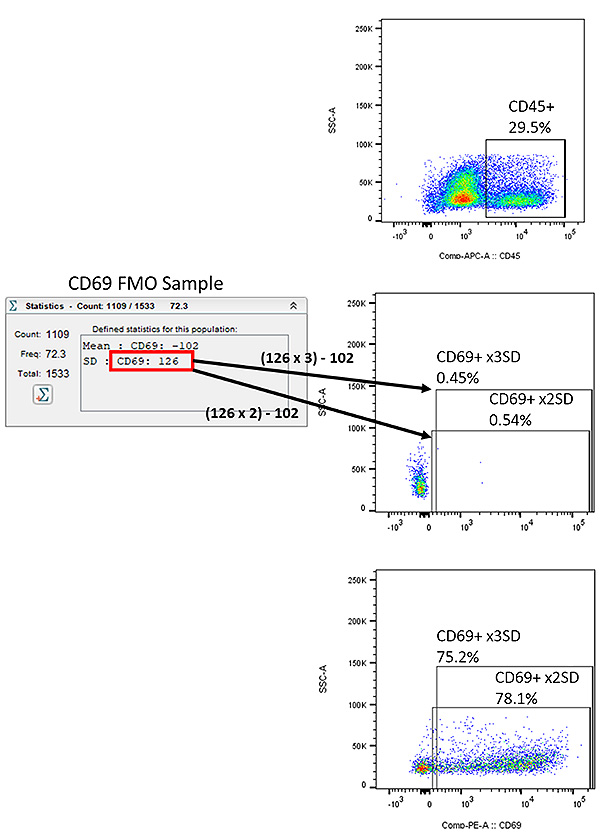
Deciding what is positive
|
This is the part where things get more subjective, and controls and consistency become even more valuable. While at times there is a clear separation between positive and negative populations, as seen below with the CD45+ population, that isn’t always the case. Having a strategy, and staying consistent is key to deciding the positive populations, especially over long running experiments where batch to batch variability will exist. With an FMO though, we can set our positive gate off a certain value of the standard deviation of the mean of the FMO population. This allows us to accurately calculate a gate position, without the guess work. In the example below, I’ve drawn gates off both 2x and 3x the SD of the mean of the FMO for CD69. You can set how strict you want this gate to be off how many SD you choose, whatever best meets the needs of your experiment. Blindly guessing a population when you could have included a proper control (FMO, non-activated, untreated, etc) is an example of cutting a corner for time that will only produce more problems down the road.
|
|
|
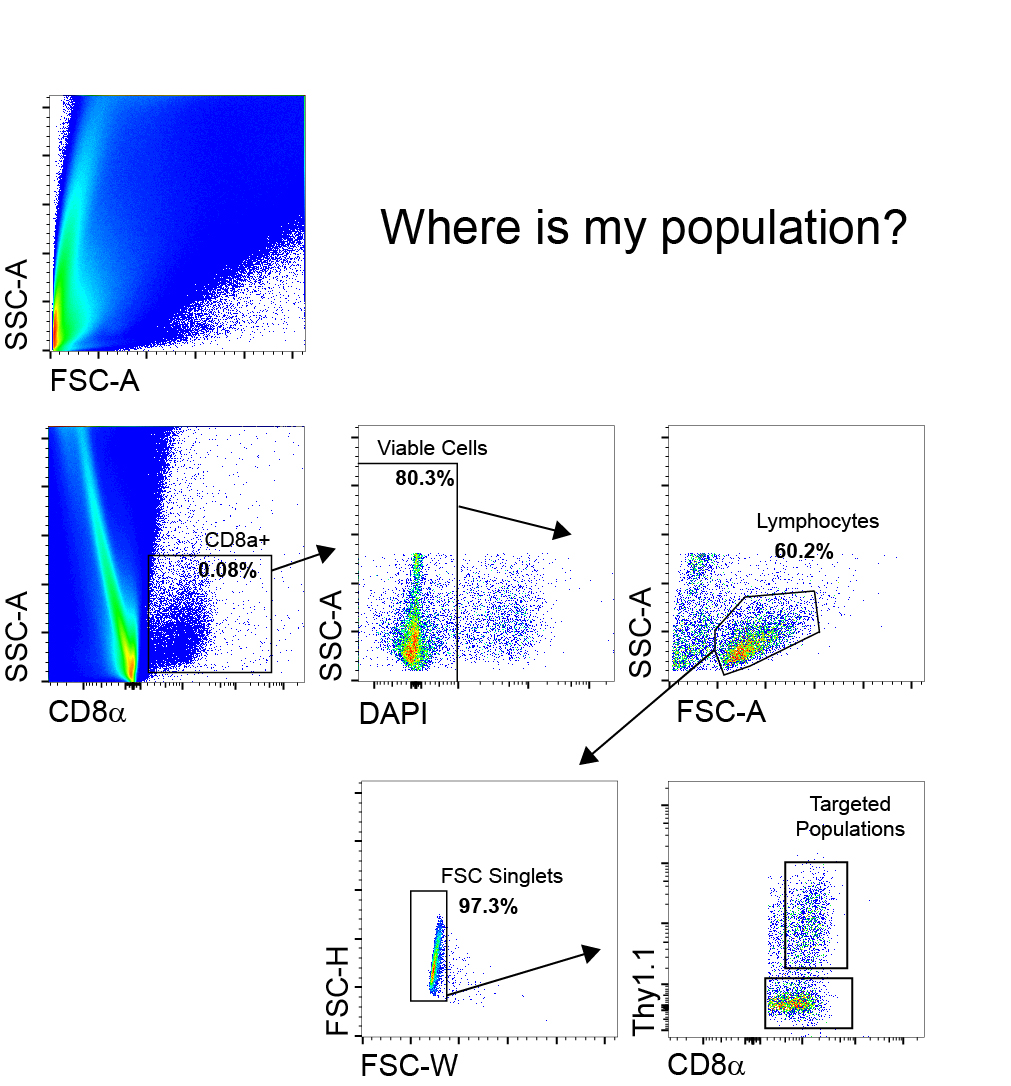
When things don’t look right
|
Sometimes the usual approach isn’t as effective. Certain samples just produce an unusual cell scatter that can leave us confused where our cells of interest are. Instead, it is better to start with a marker like CD8 for example if we are trying to pull T cells out of a messy tissue sample as seen below. From here we can work through the normal doublets exclusion and live dead gates and then to the FSCxSSC gate that we’re accustomed to.
|
|
|
Conclusions
|
This is not an exhaustive guide to gating, just a review of some necessary steps along with some advice for future experiments. While things like doublet exclusion and FSCxSSC selection will always be a necessary step in data analysis, this process is becoming increasingly more complicated. With the introduction of things like t-SNE and other high dimensional analysis techniques, existing strategies for gating populations will need to be refined. If you have any questions feel free to reach out to the FCF staff for advice on gating and data analysis.
|
|
|
|
|
|
|